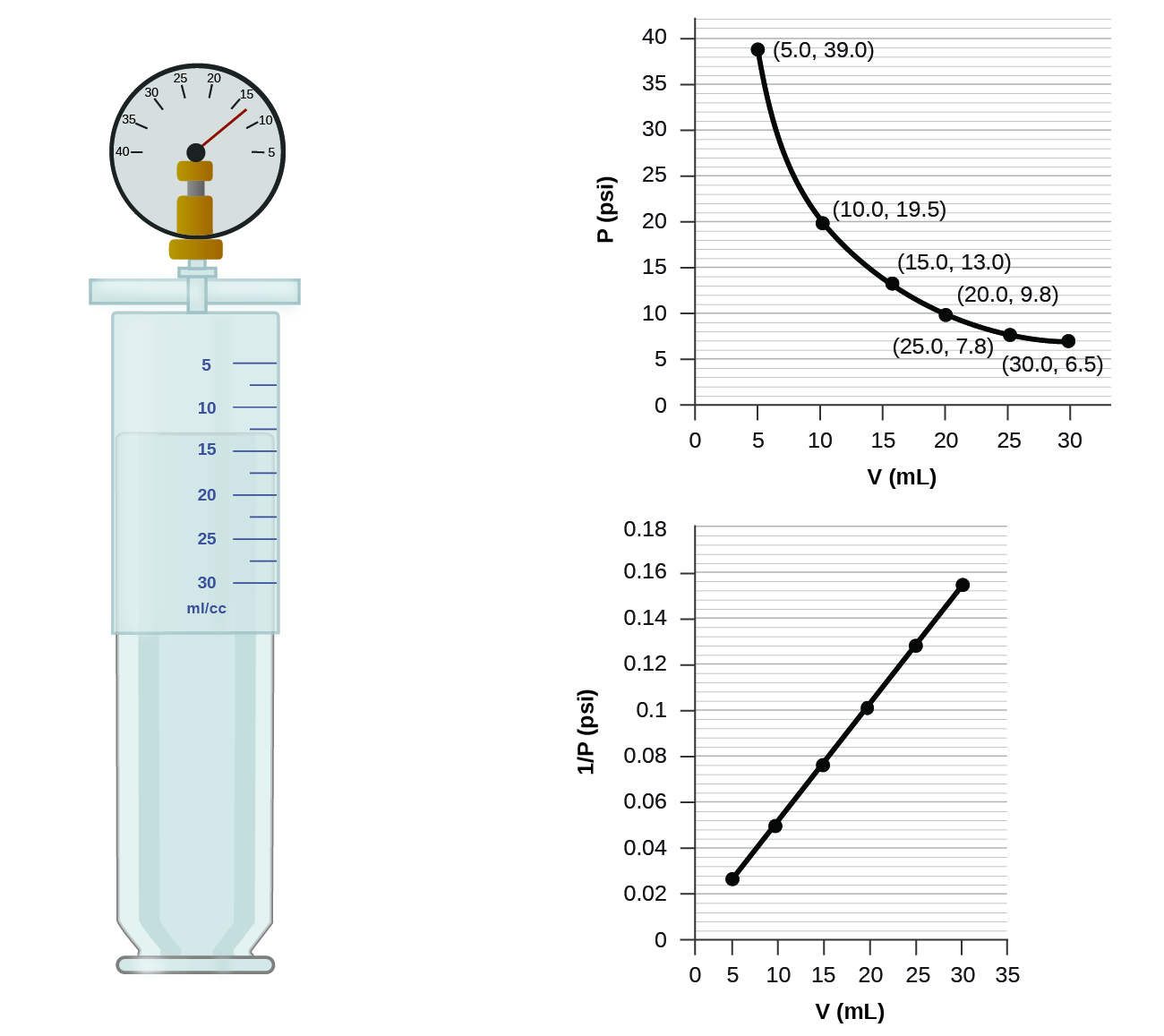
Changing Pressure Gas Volume . On earth, matter exists in one of three states: early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables. Use boyle's law to calculate. Its pressure changes to 1.93 atm. a sample of gas has an initial pressure of 2.44 atm and an initial volume of 4.01 l. the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume. define the relationship between gas volume and pressure, boyle's law. Matter in each state exhibits distinct.
from opentextbc.ca
Use boyle's law to calculate. a sample of gas has an initial pressure of 2.44 atm and an initial volume of 4.01 l. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables. Matter in each state exhibits distinct. On earth, matter exists in one of three states: Its pressure changes to 1.93 atm. define the relationship between gas volume and pressure, boyle's law. the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume. the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law).
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas
Changing Pressure Gas Volume Matter in each state exhibits distinct. Matter in each state exhibits distinct. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables. the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume. On earth, matter exists in one of three states: the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). Use boyle's law to calculate. Its pressure changes to 1.93 atm. define the relationship between gas volume and pressure, boyle's law. a sample of gas has an initial pressure of 2.44 atm and an initial volume of 4.01 l.
From www.grc.nasa.gov
Work Done by a Gas Slide Changing Pressure Gas Volume Matter in each state exhibits distinct. the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume. the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). Use boyle's law to calculate. define the relationship. Changing Pressure Gas Volume.
From www.youtube.com
1.4.6 Solve problems involving temperature, pressure and volume for an Changing Pressure Gas Volume a sample of gas has an initial pressure of 2.44 atm and an initial volume of 4.01 l. Matter in each state exhibits distinct. Use boyle's law to calculate. define the relationship between gas volume and pressure, boyle's law. Its pressure changes to 1.93 atm. early scientists explored the relationships among the pressure of a gas (p). Changing Pressure Gas Volume.
From leomi.in
Importance of Actual Mass Flow Rate in Gas Flow Measurement Changing Pressure Gas Volume On earth, matter exists in one of three states: Use boyle's law to calculate. the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount. Changing Pressure Gas Volume.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint Changing Pressure Gas Volume a sample of gas has an initial pressure of 2.44 atm and an initial volume of 4.01 l. the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume. On earth, matter exists in one of three states: Its pressure changes to 1.93 atm. the pressure of a given. Changing Pressure Gas Volume.
From cartoondealer.com
Boyle's Law Showing The Pressure And Volume Relationship Vector Changing Pressure Gas Volume Matter in each state exhibits distinct. Use boyle's law to calculate. a sample of gas has an initial pressure of 2.44 atm and an initial volume of 4.01 l. the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume. On earth, matter exists in one of three states: . Changing Pressure Gas Volume.
From www.youtube.com
Gas Pressure and Volume GCSE Physics Revision YouTube Changing Pressure Gas Volume the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). Its pressure changes to 1.93 atm. a sample of gas has an initial pressure of 2.44 atm and an initial volume of 4.01 l. On earth, matter exists in one of three states: . Changing Pressure Gas Volume.
From www.youtube.com
CHEM 101 Change in Internal Energy, Heat, and PressureVolume Work 2 Changing Pressure Gas Volume define the relationship between gas volume and pressure, boyle's law. the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume. Its pressure changes to 1.93 atm. Use boyle's law to calculate. On earth, matter exists in one of three states: Matter in each state exhibits distinct. a sample. Changing Pressure Gas Volume.
From www.britannica.com
Ideal gas Definition, Equation, Properties, & Facts Britannica Changing Pressure Gas Volume define the relationship between gas volume and pressure, boyle's law. Its pressure changes to 1.93 atm. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables. the pressure of a given amount of gas is directly proportional to its. Changing Pressure Gas Volume.
From www.shalom-education.com
Volume and Pressure in Gases GCSE Physics Revision Changing Pressure Gas Volume a sample of gas has an initial pressure of 2.44 atm and an initial volume of 4.01 l. the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). Matter in each state exhibits distinct. early scientists explored the relationships among the pressure of. Changing Pressure Gas Volume.
From www.slideserve.com
PPT Chemistry 142 Chapter 14 Chemical Equilibrium Review Changing Pressure Gas Volume Its pressure changes to 1.93 atm. the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume. the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). early scientists explored the relationships among the pressure. Changing Pressure Gas Volume.
From www.youtube.com
Ideal Gas Law Problem Given Change in Pressure and Volume Delta Changing Pressure Gas Volume a sample of gas has an initial pressure of 2.44 atm and an initial volume of 4.01 l. On earth, matter exists in one of three states: Matter in each state exhibits distinct. the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume. define the relationship between gas. Changing Pressure Gas Volume.
From www.youtube.com
11.6 The Combined Gas Law Pressure, Volume, & Temperature YouTube Changing Pressure Gas Volume the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables. On earth, matter exists in one of three states: a. Changing Pressure Gas Volume.
From www.youtube.com
Pressure, Volume and Temperature Relationships Chemistry Tutorial Changing Pressure Gas Volume the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). a sample of gas has an initial pressure of 2.44 atm and an initial volume of 4.01 l. Use boyle's law to calculate. On earth, matter exists in one of three states: early. Changing Pressure Gas Volume.
From www.slideserve.com
PPT Chapter 14 Gas Laws PowerPoint Presentation, free download ID Changing Pressure Gas Volume Use boyle's law to calculate. a sample of gas has an initial pressure of 2.44 atm and an initial volume of 4.01 l. On earth, matter exists in one of three states: early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the. Changing Pressure Gas Volume.
From www.youtube.com
Work Done by a Gas During Pressure and Volume Change YouTube Changing Pressure Gas Volume early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables. a sample of gas has an initial pressure of 2.44 atm and an initial volume of 4.01 l. Use boyle's law to calculate. Its pressure changes to 1.93 atm. On. Changing Pressure Gas Volume.
From www.slideserve.com
PPT Unit 5 Gases More Gas Laws Charles’s Law and Boyle’s Law Changing Pressure Gas Volume Matter in each state exhibits distinct. the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume. On earth, matter exists in one of three states: early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two. Changing Pressure Gas Volume.
From www.tes.com
GCSE AQA Physics P6.7 Pressure and Volume Teaching Resources Changing Pressure Gas Volume early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables. Its pressure changes to 1.93 atm. Matter in each state exhibits distinct. the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the. Changing Pressure Gas Volume.
From opentextbc.ca
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Changing Pressure Gas Volume Its pressure changes to 1.93 atm. a sample of gas has an initial pressure of 2.44 atm and an initial volume of 4.01 l. On earth, matter exists in one of three states: the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume. the pressure of a given. Changing Pressure Gas Volume.
From www.edplace.com
Understand Gas Pressure Worksheet EdPlace Changing Pressure Gas Volume On earth, matter exists in one of three states: the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). Matter in each state exhibits distinct. a sample of gas has an initial pressure of 2.44 atm and an initial volume of 4.01 l. . Changing Pressure Gas Volume.
From masterconceptsinchemistry.com
What’s the relationship between pressure and volume of gas? Core Changing Pressure Gas Volume On earth, matter exists in one of three states: define the relationship between gas volume and pressure, boyle's law. Its pressure changes to 1.93 atm. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables. the pressure of a. Changing Pressure Gas Volume.
From www.youtube.com
Ideal Gas Pressure and Volume Dependence on Temperature YouTube Changing Pressure Gas Volume On earth, matter exists in one of three states: Use boyle's law to calculate. the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume. define the relationship between gas volume and pressure, boyle's law. early scientists explored the relationships among the pressure of a gas (p) and its. Changing Pressure Gas Volume.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo Changing Pressure Gas Volume define the relationship between gas volume and pressure, boyle's law. a sample of gas has an initial pressure of 2.44 atm and an initial volume of 4.01 l. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables. Matter. Changing Pressure Gas Volume.
From opentextbc.ca
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Changing Pressure Gas Volume a sample of gas has an initial pressure of 2.44 atm and an initial volume of 4.01 l. Use boyle's law to calculate. the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume. define the relationship between gas volume and pressure, boyle's law. Its pressure changes to 1.93. Changing Pressure Gas Volume.
From www.youtube.com
Combined Gas Law Pressure, Volume and Temperature Straight Science Changing Pressure Gas Volume define the relationship between gas volume and pressure, boyle's law. the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). Its pressure changes to 1.93 atm. On earth, matter exists in one of three states: early scientists explored the relationships among the pressure. Changing Pressure Gas Volume.
From www.dreamstime.com
Ideal Gas Law. Boyles Law Pressure Volume Relationship in Gases Stock Changing Pressure Gas Volume Use boyle's law to calculate. a sample of gas has an initial pressure of 2.44 atm and an initial volume of 4.01 l. On earth, matter exists in one of three states: define the relationship between gas volume and pressure, boyle's law. Matter in each state exhibits distinct. Its pressure changes to 1.93 atm. the pressure of. Changing Pressure Gas Volume.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Changing Pressure Gas Volume Use boyle's law to calculate. a sample of gas has an initial pressure of 2.44 atm and an initial volume of 4.01 l. On earth, matter exists in one of three states: Matter in each state exhibits distinct. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount. Changing Pressure Gas Volume.
From www.grc.nasa.gov
Work Done by a Gas Changing Pressure Gas Volume Matter in each state exhibits distinct. a sample of gas has an initial pressure of 2.44 atm and an initial volume of 4.01 l. the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). On earth, matter exists in one of three states: . Changing Pressure Gas Volume.
From www.nagwa.com
Question Video Identifying the Relationship between the Pressure and Changing Pressure Gas Volume a sample of gas has an initial pressure of 2.44 atm and an initial volume of 4.01 l. the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume. the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does. Changing Pressure Gas Volume.
From www.chegg.com
Solved A fixed volume of an ideal gas held at a constant Changing Pressure Gas Volume Use boyle's law to calculate. Matter in each state exhibits distinct. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables. the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume.. Changing Pressure Gas Volume.
From tardigrade.in
In diagrams ( 1 to 4 ), variation of volume with changing pressure is Changing Pressure Gas Volume the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume. On earth, matter exists in one of three states: Its pressure changes to 1.93 atm. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two. Changing Pressure Gas Volume.
From www.nesscopressure.com.au
What is air pressure? Nessco Pressure Systems Changing Pressure Gas Volume a sample of gas has an initial pressure of 2.44 atm and an initial volume of 4.01 l. On earth, matter exists in one of three states: the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume. Its pressure changes to 1.93 atm. the pressure of a given. Changing Pressure Gas Volume.
From studyrocket.co.uk
Turning Forces and Pressure GCSE Physics AQA Revision Study Rocket Changing Pressure Gas Volume early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables. Its pressure changes to 1.93 atm. Use boyle's law to calculate. Matter in each state exhibits distinct. define the relationship between gas volume and pressure, boyle's law. the pressure. Changing Pressure Gas Volume.
From www.slideserve.com
PPT Chapter 14 Properties of Gases PowerPoint Presentation, free Changing Pressure Gas Volume define the relationship between gas volume and pressure, boyle's law. the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). Use boyle's law to calculate. a sample of gas has an initial pressure of 2.44 atm and an initial volume of 4.01 l.. Changing Pressure Gas Volume.
From www.nagwa.com
Question Video Calculating the Change of the Volume of a Gas under Changing Pressure Gas Volume Its pressure changes to 1.93 atm. On earth, matter exists in one of three states: the pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). Use boyle's law to calculate. early scientists explored the relationships among the pressure of a gas (p) and its. Changing Pressure Gas Volume.
From www.youtube.com
Pressure, Volume, Temperature and Mole Relationships YouTube Changing Pressure Gas Volume Its pressure changes to 1.93 atm. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables. a sample of gas has an initial pressure of 2.44 atm and an initial volume of 4.01 l. Use boyle's law to calculate. . Changing Pressure Gas Volume.