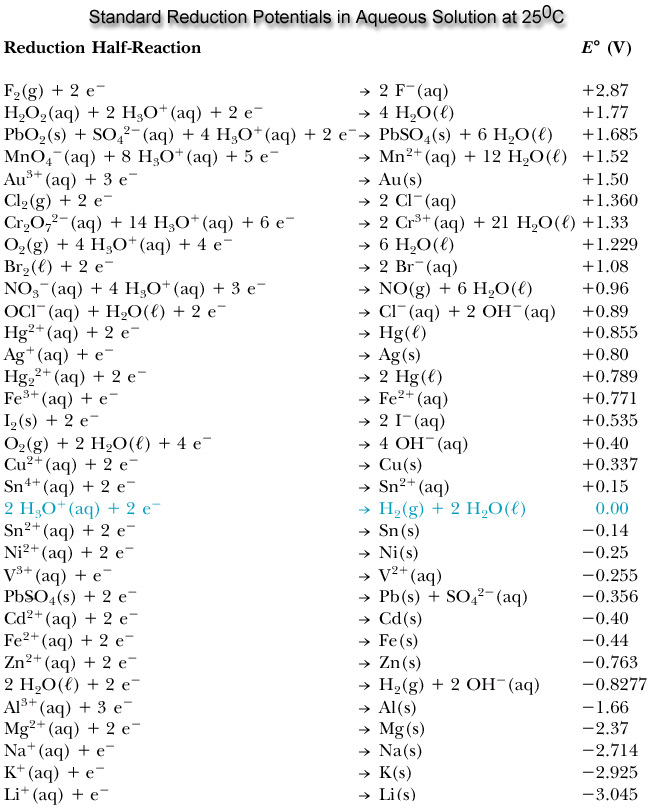
Standard Reduction Potential Chart . Use standard reduction potentials to. standard electrode (reduction) potentials in aqueous solution at 25 °c. the following table provides e o for selected reduction reactions. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. Values are from the following sources: Use standard reduction potentials to determine the. the following table provides e o and e o ´ values for selected reduction reactions. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. 45 rows standard electrode potentials in aqueous solution at 25°c.
from ch302.cm.utexas.edu
standard electrode (reduction) potentials in aqueous solution at 25 °c. Use standard reduction potentials to. Values are from the following sources: the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. the following table provides e o for selected reduction reactions. 45 rows standard electrode potentials in aqueous solution at 25°c. Use standard reduction potentials to determine the. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. the following table provides e o and e o ´ values for selected reduction reactions.
Electrochemistry_Reduction_Potentials
Standard Reduction Potential Chart Use standard reduction potentials to. 45 rows standard electrode potentials in aqueous solution at 25°c. the following table provides e o for selected reduction reactions. Use standard reduction potentials to. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. Values are from the following sources: the following table provides e o and e o ´ values for selected reduction reactions. standard electrode (reduction) potentials in aqueous solution at 25 °c. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. Use standard reduction potentials to determine the.
From f15.beauty
Standard Reduction Potential Table Standard Reduction Potential Chart the following table provides e o for selected reduction reactions. standard electrode (reduction) potentials in aqueous solution at 25 °c. the following table provides e o and e o ´ values for selected reduction reactions. Use standard reduction potentials to. Use standard reduction potentials to determine the. Values are from the following sources: the standard reduction. Standard Reduction Potential Chart.
From exyzzodqu.blob.core.windows.net
Standard Reduction Potential Values Of Three Metallic at Charles Love blog Standard Reduction Potential Chart standard electrode (reduction) potentials in aqueous solution at 25 °c. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. Use standard reduction potentials to. Use standard reduction potentials to determine the. Values are from the following sources: the following table provides e o for. Standard Reduction Potential Chart.
From pressbooks.uiowa.edu
Topic 11 Appendix A Standard Reduction Potentials at 25ºC CHEM 1120 Standard Reduction Potential Chart Values are from the following sources: the following table provides e o and e o ´ values for selected reduction reactions. standard electrode (reduction) potentials in aqueous solution at 25 °c. Use standard reduction potentials to determine the. 45 rows standard electrode potentials in aqueous solution at 25°c. Use standard reduction potentials to. the standard reduction. Standard Reduction Potential Chart.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Chart the following table provides e o and e o ´ values for selected reduction reactions. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. Values are from the following sources: the values of standard electrode potentials are given in the table in volts relative to the. Standard Reduction Potential Chart.
From ar.inspiredpencil.com
Standard Reduction Potential Table Standard Reduction Potential Chart the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. the following table provides e o for selected reduction reactions. Values are from the following. Standard Reduction Potential Chart.
From www.researchgate.net
Gibbs free energy changes and standard reduction potentials at 25 • C Standard Reduction Potential Chart Use standard reduction potentials to. 45 rows standard electrode potentials in aqueous solution at 25°c. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. the following table provides e o for selected reduction reactions. Use standard reduction potentials to determine the. Values are from. Standard Reduction Potential Chart.
From jonathancoles.z13.web.core.windows.net
Standard Reduction Potential Chart Standard Reduction Potential Chart the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. the following table provides e o for selected reduction reactions. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. 45 rows standard electrode. Standard Reduction Potential Chart.
From www.tpsearchtool.com
Table Of Standard Reduction Potentialspdf Table Of Standard Images Standard Reduction Potential Chart standard electrode (reduction) potentials in aqueous solution at 25 °c. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. the following table provides e o and e o ´ values for selected reduction reactions. 45 rows standard electrode potentials in aqueous solution at 25°c. . Standard Reduction Potential Chart.
From labbyag.es
Reduction Potential Chart Labb by AG Standard Reduction Potential Chart the following table provides e o for selected reduction reactions. Use standard reduction potentials to determine the. 45 rows standard electrode potentials in aqueous solution at 25°c. Values are from the following sources: standard electrode (reduction) potentials in aqueous solution at 25 °c. Use standard reduction potentials to. the standard reduction potential is the tendency for. Standard Reduction Potential Chart.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Chart the following table provides e o for selected reduction reactions. Use standard reduction potentials to determine the. 45 rows standard electrode potentials in aqueous solution at 25°c. Values are from the following sources: Use standard reduction potentials to. the following table provides e o and e o ´ values for selected reduction reactions. standard electrode (reduction). Standard Reduction Potential Chart.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Chart 45 rows standard electrode potentials in aqueous solution at 25°c. standard electrode (reduction) potentials in aqueous solution at 25 °c. Use standard reduction potentials to determine the. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. the following table provides e o for selected reduction. Standard Reduction Potential Chart.
From rachelbrowne.z13.web.core.windows.net
Standard Reduction Potential Chart Standard Reduction Potential Chart the following table provides e o for selected reduction reactions. standard electrode (reduction) potentials in aqueous solution at 25 °c. the following table provides e o and e o ´ values for selected reduction reactions. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. Use. Standard Reduction Potential Chart.
From www.scribd.com
Standard Redox Potential Table.pdf Electrochemistry Redox Standard Reduction Potential Chart 45 rows standard electrode potentials in aqueous solution at 25°c. the following table provides e o and e o ´ values for selected reduction reactions. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. standard electrode (reduction) potentials in aqueous solution at 25 °c. Use. Standard Reduction Potential Chart.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Chart the following table provides e o and e o ´ values for selected reduction reactions. Values are from the following sources: the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. Use standard reduction potentials to determine the. standard electrode (reduction) potentials in aqueous solution at 25. Standard Reduction Potential Chart.
From rayb78.github.io
Standard Reduction Potentials Chart Standard Reduction Potential Chart standard electrode (reduction) potentials in aqueous solution at 25 °c. Use standard reduction potentials to. Values are from the following sources: the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. the following table provides e o for selected reduction reactions. the following table provides e. Standard Reduction Potential Chart.
From ar.inspiredpencil.com
Standard Reduction Potential Table Standard Reduction Potential Chart Values are from the following sources: the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. the following table provides e o for selected reduction reactions. 45 rows standard electrode potentials in aqueous solution at 25°c. the standard reduction potential is the tendency for. Standard Reduction Potential Chart.
From www.vrogue.co
Standard Reduction Potential Table Lydiarillynn vrogue.co Standard Reduction Potential Chart Use standard reduction potentials to determine the. Use standard reduction potentials to. the following table provides e o and e o ´ values for selected reduction reactions. standard electrode (reduction) potentials in aqueous solution at 25 °c. 45 rows standard electrode potentials in aqueous solution at 25°c. the standard reduction potential is the tendency for a. Standard Reduction Potential Chart.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Chart Values are from the following sources: Use standard reduction potentials to. standard electrode (reduction) potentials in aqueous solution at 25 °c. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. the following table provides e o and e o ´ values for selected reduction reactions. Use. Standard Reduction Potential Chart.
From ch302.cm.utexas.edu
Electrochemistry_Reduction_Potentials Standard Reduction Potential Chart standard electrode (reduction) potentials in aqueous solution at 25 °c. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. Use standard reduction potentials to. Use standard reduction potentials to determine the. the following table provides e o and e o ´ values for selected reduction reactions.. Standard Reduction Potential Chart.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Chart 45 rows standard electrode potentials in aqueous solution at 25°c. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. Use standard reduction potentials to determine the. Use standard reduction potentials to. Values are from the following sources: standard electrode (reduction) potentials in aqueous solution. Standard Reduction Potential Chart.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Chart standard electrode (reduction) potentials in aqueous solution at 25 °c. Values are from the following sources: Use standard reduction potentials to determine the. the following table provides e o and e o ´ values for selected reduction reactions. Use standard reduction potentials to. 45 rows standard electrode potentials in aqueous solution at 25°c. the values of. Standard Reduction Potential Chart.
From www.tpsearchtool.com
Appendix 4 Standard Reduction Potentials First Year General Chemistry Standard Reduction Potential Chart 45 rows standard electrode potentials in aqueous solution at 25°c. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. standard electrode (reduction) potentials in aqueous solution at 25 °c. Values are from the following sources: Use standard reduction potentials to determine the. Use standard. Standard Reduction Potential Chart.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Chart Use standard reduction potentials to. Use standard reduction potentials to determine the. the following table provides e o and e o ´ values for selected reduction reactions. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. 45 rows standard electrode potentials in aqueous solution at 25°c.. Standard Reduction Potential Chart.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Chart Use standard reduction potentials to. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. Use standard reduction potentials to determine the. standard electrode (reduction). Standard Reduction Potential Chart.
From rayb78.github.io
Standard Reduction Potentials Chart Standard Reduction Potential Chart Use standard reduction potentials to determine the. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. Use standard reduction potentials to. the following table provides e o for selected reduction reactions. the following table provides e o and e o ´ values for selected reduction reactions.. Standard Reduction Potential Chart.
From pveducation.org
Standard Potential PVEducation Standard Reduction Potential Chart the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. Values are from the following sources: the following table provides e o for selected reduction. Standard Reduction Potential Chart.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Chart standard electrode (reduction) potentials in aqueous solution at 25 °c. Values are from the following sources: the following table provides e o for selected reduction reactions. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. the standard reduction potential is the tendency for. Standard Reduction Potential Chart.
From www.chegg.com
Solved Standard Reduction Potential Table (at 25°C, 101kPa, Standard Reduction Potential Chart the following table provides e o for selected reduction reactions. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. the following table provides e o and e o ´ values for selected reduction reactions. Values are from the following sources: standard electrode (reduction) potentials in. Standard Reduction Potential Chart.
From jonathancoles.z13.web.core.windows.net
Standard Reduction Potential Chart Standard Reduction Potential Chart standard electrode (reduction) potentials in aqueous solution at 25 °c. the following table provides e o and e o ´ values for selected reduction reactions. Use standard reduction potentials to. Use standard reduction potentials to determine the. Values are from the following sources: the following table provides e o for selected reduction reactions. 45 rows standard. Standard Reduction Potential Chart.
From www.scribd.com
Standard Reduction Potentials Data Extended pdf Standard Reduction Potential Chart standard electrode (reduction) potentials in aqueous solution at 25 °c. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. Use standard reduction potentials to determine the. Values are from the following sources: the following table provides e o for selected reduction reactions. 45 rows standard. Standard Reduction Potential Chart.
From rayb78.github.io
Standard Reduction Potentials Chart Standard Reduction Potential Chart 45 rows standard electrode potentials in aqueous solution at 25°c. Use standard reduction potentials to. the following table provides e o and e o ´ values for selected reduction reactions. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. the following table provides e o. Standard Reduction Potential Chart.
From www.vrogue.co
Standard Reduction Potential Table Reduction Potentia vrogue.co Standard Reduction Potential Chart the following table provides e o and e o ´ values for selected reduction reactions. Use standard reduction potentials to determine the. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. Use standard reduction potentials to. the standard reduction potential is the tendency for. Standard Reduction Potential Chart.
From www.flinnsci.ca
Standard Reduction Potential Charts for Chemistry Standard Reduction Potential Chart Values are from the following sources: 45 rows standard electrode potentials in aqueous solution at 25°c. the following table provides e o for selected reduction reactions. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. Use standard reduction potentials to. Use standard reduction potentials. Standard Reduction Potential Chart.
From www.tpsearchtool.com
Table Of Standard Reduction Potentialspdf Table Of Standard Images Standard Reduction Potential Chart Values are from the following sources: Use standard reduction potentials to determine the. standard electrode (reduction) potentials in aqueous solution at 25 °c. Use standard reduction potentials to. the following table provides e o and e o ´ values for selected reduction reactions. the following table provides e o for selected reduction reactions. the standard reduction. Standard Reduction Potential Chart.
From www.tpsearchtool.com
Standard Redox Potential Tablepdf Electrochemistry Redox Images Standard Reduction Potential Chart the following table provides e o and e o ´ values for selected reduction reactions. Values are from the following sources: the following table provides e o for selected reduction reactions. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. Use standard reduction potentials to. . Standard Reduction Potential Chart.