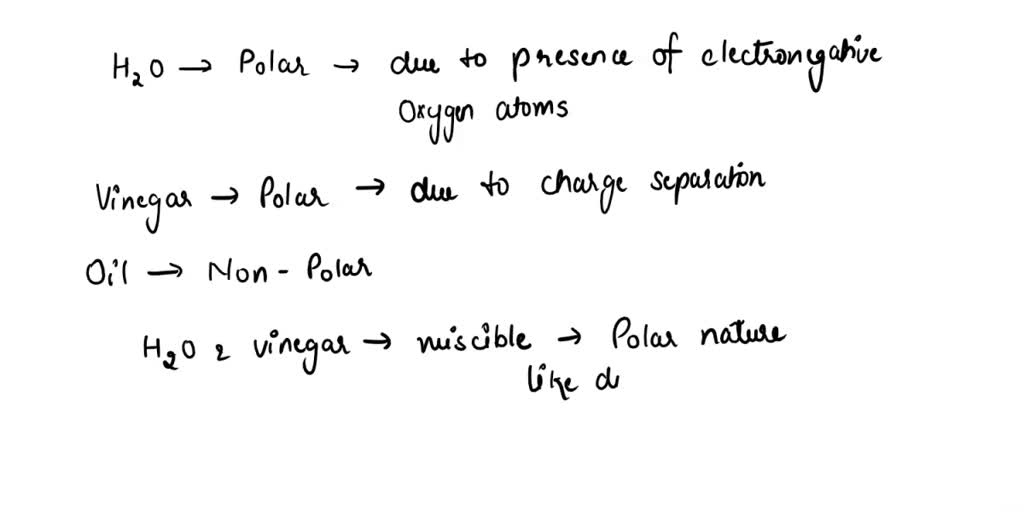
Vinegar And Oil Are Immiscible . The primary reason for the immiscibility of. Immiscibility occurs due to the difference in polarity and. Oil is nonpolar, while vinegar is polar. These two substances are immiscible, meaning they cannot form a. the mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. what is the reason behind the immiscibility of oil and vinegar? yes, you can mix vinegar and oil temporarily by creating an emulsion. oil and vinegar are immiscible because they have different polarities. the simple answer is no, vinegar and oil do not mix. oils repel polar molecules such as those found in vinegar. Emulsification involves blending two immiscible. let’s delve into the scientific explanation behind their refusal to blend.
from www.numerade.com
Immiscibility occurs due to the difference in polarity and. These two substances are immiscible, meaning they cannot form a. Oil is nonpolar, while vinegar is polar. what is the reason behind the immiscibility of oil and vinegar? the simple answer is no, vinegar and oil do not mix. oil and vinegar are immiscible because they have different polarities. let’s delve into the scientific explanation behind their refusal to blend. Emulsification involves blending two immiscible. yes, you can mix vinegar and oil temporarily by creating an emulsion. the mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers.
SOLVED Using the concept of bond polarity and solubility, explain why
Vinegar And Oil Are Immiscible Oil is nonpolar, while vinegar is polar. let’s delve into the scientific explanation behind their refusal to blend. These two substances are immiscible, meaning they cannot form a. Immiscibility occurs due to the difference in polarity and. the mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. The primary reason for the immiscibility of. Oil is nonpolar, while vinegar is polar. oil and vinegar are immiscible because they have different polarities. oils repel polar molecules such as those found in vinegar. what is the reason behind the immiscibility of oil and vinegar? yes, you can mix vinegar and oil temporarily by creating an emulsion. Emulsification involves blending two immiscible. the simple answer is no, vinegar and oil do not mix.
From studiousguy.com
8 Miscible Liquids Examples in Daily Life StudiousGuy Vinegar And Oil Are Immiscible Oil is nonpolar, while vinegar is polar. what is the reason behind the immiscibility of oil and vinegar? the mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. yes, you can mix vinegar and oil temporarily by creating an emulsion. let’s delve into the scientific explanation behind their refusal. Vinegar And Oil Are Immiscible.
From www.alamy.com
Oil and vinegar are immiscible hires stock photography and images Alamy Vinegar And Oil Are Immiscible the mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. the simple answer is no, vinegar and oil do not mix. what is the reason behind the immiscibility of oil and vinegar? Oil is nonpolar, while vinegar is polar. The primary reason for the immiscibility of. Immiscibility occurs due to. Vinegar And Oil Are Immiscible.
From focusedcollection.com
Oil and vinegar on glass plate against white background. — science Vinegar And Oil Are Immiscible oil and vinegar are immiscible because they have different polarities. what is the reason behind the immiscibility of oil and vinegar? yes, you can mix vinegar and oil temporarily by creating an emulsion. These two substances are immiscible, meaning they cannot form a. Oil is nonpolar, while vinegar is polar. Immiscibility occurs due to the difference in. Vinegar And Oil Are Immiscible.
From www.alamy.com
An illustration of immiscible liquids water and oil in an emulsion Vinegar And Oil Are Immiscible Immiscibility occurs due to the difference in polarity and. Emulsification involves blending two immiscible. These two substances are immiscible, meaning they cannot form a. the simple answer is no, vinegar and oil do not mix. The primary reason for the immiscibility of. let’s delve into the scientific explanation behind their refusal to blend. oil and vinegar are. Vinegar And Oil Are Immiscible.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Miscible; immiscible Vinegar And Oil Are Immiscible oil and vinegar are immiscible because they have different polarities. Immiscibility occurs due to the difference in polarity and. These two substances are immiscible, meaning they cannot form a. The primary reason for the immiscibility of. the mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. yes, you can mix. Vinegar And Oil Are Immiscible.
From www.alamy.com
Immiscible liquids. Sequence showing a flask containing vegetable oil Vinegar And Oil Are Immiscible Emulsification involves blending two immiscible. Immiscibility occurs due to the difference in polarity and. the simple answer is no, vinegar and oil do not mix. the mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. oils repel polar molecules such as those found in vinegar. oil and vinegar are. Vinegar And Oil Are Immiscible.
From slideplayer.com
Chapter 7 Solutions and Other Mixtures ppt download Vinegar And Oil Are Immiscible yes, you can mix vinegar and oil temporarily by creating an emulsion. Oil is nonpolar, while vinegar is polar. oil and vinegar are immiscible because they have different polarities. The primary reason for the immiscibility of. These two substances are immiscible, meaning they cannot form a. the mixing of oil and vinegar produces a temporary mixture that. Vinegar And Oil Are Immiscible.
From slideplayer.com
Chapter 15 Solutions. ppt download Vinegar And Oil Are Immiscible let’s delve into the scientific explanation behind their refusal to blend. Immiscibility occurs due to the difference in polarity and. The primary reason for the immiscibility of. the mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. Oil is nonpolar, while vinegar is polar. oil and vinegar are immiscible because. Vinegar And Oil Are Immiscible.
From www.slideserve.com
PPT Separation of Mixtures PowerPoint Presentation, free download Vinegar And Oil Are Immiscible Oil is nonpolar, while vinegar is polar. oils repel polar molecules such as those found in vinegar. The primary reason for the immiscibility of. let’s delve into the scientific explanation behind their refusal to blend. the simple answer is no, vinegar and oil do not mix. Emulsification involves blending two immiscible. what is the reason behind. Vinegar And Oil Are Immiscible.
From www.numerade.com
SOLVED using the concept of bond polarity solubility explain why water Vinegar And Oil Are Immiscible Immiscibility occurs due to the difference in polarity and. oils repel polar molecules such as those found in vinegar. oil and vinegar are immiscible because they have different polarities. yes, you can mix vinegar and oil temporarily by creating an emulsion. what is the reason behind the immiscibility of oil and vinegar? Emulsification involves blending two. Vinegar And Oil Are Immiscible.
From www.thoughtco.com
Immiscible Definition and Examples (Chemistry) Vinegar And Oil Are Immiscible These two substances are immiscible, meaning they cannot form a. Immiscibility occurs due to the difference in polarity and. the simple answer is no, vinegar and oil do not mix. oils repel polar molecules such as those found in vinegar. Oil is nonpolar, while vinegar is polar. The primary reason for the immiscibility of. the mixing of. Vinegar And Oil Are Immiscible.
From slideplayer.com
Bellwork 1. What do you remember about the difference between Vinegar And Oil Are Immiscible let’s delve into the scientific explanation behind their refusal to blend. the simple answer is no, vinegar and oil do not mix. Emulsification involves blending two immiscible. Immiscibility occurs due to the difference in polarity and. oil and vinegar are immiscible because they have different polarities. Oil is nonpolar, while vinegar is polar. These two substances are. Vinegar And Oil Are Immiscible.
From www.slideserve.com
PPT Chemistry B Intro to Mixtures PowerPoint Presentation, free Vinegar And Oil Are Immiscible let’s delve into the scientific explanation behind their refusal to blend. oils repel polar molecules such as those found in vinegar. what is the reason behind the immiscibility of oil and vinegar? Emulsification involves blending two immiscible. the mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. Oil is. Vinegar And Oil Are Immiscible.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Miscible; immiscible Vinegar And Oil Are Immiscible oils repel polar molecules such as those found in vinegar. the mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. Oil is nonpolar, while vinegar is polar. the simple answer is no, vinegar and oil do not mix. yes, you can mix vinegar and oil temporarily by creating an. Vinegar And Oil Are Immiscible.
From www.numerade.com
SOLVED Using the concept of bond polarity and solubility, explain why Vinegar And Oil Are Immiscible let’s delve into the scientific explanation behind their refusal to blend. Emulsification involves blending two immiscible. yes, you can mix vinegar and oil temporarily by creating an emulsion. Immiscibility occurs due to the difference in polarity and. oils repel polar molecules such as those found in vinegar. oil and vinegar are immiscible because they have different. Vinegar And Oil Are Immiscible.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Miscible; immiscible Vinegar And Oil Are Immiscible These two substances are immiscible, meaning they cannot form a. Immiscibility occurs due to the difference in polarity and. The primary reason for the immiscibility of. yes, you can mix vinegar and oil temporarily by creating an emulsion. oil and vinegar are immiscible because they have different polarities. the mixing of oil and vinegar produces a temporary. Vinegar And Oil Are Immiscible.
From www.numerade.com
SOLVED. Using the concept of bond polarity and solubility, explain why Vinegar And Oil Are Immiscible oils repel polar molecules such as those found in vinegar. let’s delve into the scientific explanation behind their refusal to blend. the mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. yes, you can mix vinegar and oil temporarily by creating an emulsion. The primary reason for the immiscibility. Vinegar And Oil Are Immiscible.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Miscible; immiscible Vinegar And Oil Are Immiscible the mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. Emulsification involves blending two immiscible. oils repel polar molecules such as those found in vinegar. Immiscibility occurs due to the difference in polarity and. what is the reason behind the immiscibility of oil and vinegar? yes, you can mix. Vinegar And Oil Are Immiscible.
From www.thoughtco.com
Immiscible Definition and Example Vinegar And Oil Are Immiscible what is the reason behind the immiscibility of oil and vinegar? Immiscibility occurs due to the difference in polarity and. the mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. let’s delve into the scientific explanation behind their refusal to blend. oil and vinegar are immiscible because they have. Vinegar And Oil Are Immiscible.
From slidetodoc.com
Solubility the concentration of a saturated solution at Vinegar And Oil Are Immiscible yes, you can mix vinegar and oil temporarily by creating an emulsion. the simple answer is no, vinegar and oil do not mix. let’s delve into the scientific explanation behind their refusal to blend. Immiscibility occurs due to the difference in polarity and. oils repel polar molecules such as those found in vinegar. These two substances. Vinegar And Oil Are Immiscible.
From depositphotos.com
Physics. Immiscible fluids, oil and water. Series. 2 0f 4. Stock Photo Vinegar And Oil Are Immiscible Oil is nonpolar, while vinegar is polar. oil and vinegar are immiscible because they have different polarities. These two substances are immiscible, meaning they cannot form a. the mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. Emulsification involves blending two immiscible. the simple answer is no, vinegar and oil. Vinegar And Oil Are Immiscible.
From www.gourmethousewife.com
Oil and Vinegar Dressing The Gourmet Housewife Vinegar And Oil Are Immiscible Immiscibility occurs due to the difference in polarity and. the mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. the simple answer is no, vinegar and oil do not mix. Oil is nonpolar, while vinegar is polar. yes, you can mix vinegar and oil temporarily by creating an emulsion. . Vinegar And Oil Are Immiscible.
From slideplayer.com
Chapter 13 “Solutions”. ppt download Vinegar And Oil Are Immiscible Emulsification involves blending two immiscible. what is the reason behind the immiscibility of oil and vinegar? These two substances are immiscible, meaning they cannot form a. The primary reason for the immiscibility of. oil and vinegar are immiscible because they have different polarities. the simple answer is no, vinegar and oil do not mix. Oil is nonpolar,. Vinegar And Oil Are Immiscible.
From www.sciencephoto.com
Oil and vinegar Stock Image C050/4846 Science Photo Library Vinegar And Oil Are Immiscible oil and vinegar are immiscible because they have different polarities. yes, you can mix vinegar and oil temporarily by creating an emulsion. what is the reason behind the immiscibility of oil and vinegar? These two substances are immiscible, meaning they cannot form a. The primary reason for the immiscibility of. oils repel polar molecules such as. Vinegar And Oil Are Immiscible.
From www.slideshare.net
Chemistry Chp 16 Solutions PowerPoint (shortened) Vinegar And Oil Are Immiscible oils repel polar molecules such as those found in vinegar. the mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. yes, you can mix vinegar and oil temporarily by creating an emulsion. The primary reason for the immiscibility of. let’s delve into the scientific explanation behind their refusal to. Vinegar And Oil Are Immiscible.
From www.slideserve.com
PPT DOWNLOAD/PDF Vinegar and Oil Nature's magic the ultimate Vinegar And Oil Are Immiscible yes, you can mix vinegar and oil temporarily by creating an emulsion. Emulsification involves blending two immiscible. Immiscibility occurs due to the difference in polarity and. oils repel polar molecules such as those found in vinegar. These two substances are immiscible, meaning they cannot form a. the simple answer is no, vinegar and oil do not mix.. Vinegar And Oil Are Immiscible.
From slideplayer.com
Solution Chemistry Part I Notes 8b ppt download Vinegar And Oil Are Immiscible what is the reason behind the immiscibility of oil and vinegar? let’s delve into the scientific explanation behind their refusal to blend. oils repel polar molecules such as those found in vinegar. yes, you can mix vinegar and oil temporarily by creating an emulsion. Oil is nonpolar, while vinegar is polar. Emulsification involves blending two immiscible.. Vinegar And Oil Are Immiscible.
From www.sciencephoto.com
Oil and vinegar, 2 of 2 Stock Image C050/4776 Science Photo Library Vinegar And Oil Are Immiscible the simple answer is no, vinegar and oil do not mix. Emulsification involves blending two immiscible. The primary reason for the immiscibility of. what is the reason behind the immiscibility of oil and vinegar? Immiscibility occurs due to the difference in polarity and. These two substances are immiscible, meaning they cannot form a. the mixing of oil. Vinegar And Oil Are Immiscible.
From slideplayer.com
agitation) _____________ ppt download Vinegar And Oil Are Immiscible the mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. Immiscibility occurs due to the difference in polarity and. oils repel polar molecules such as those found in vinegar. Emulsification involves blending two immiscible. what is the reason behind the immiscibility of oil and vinegar? let’s delve into the. Vinegar And Oil Are Immiscible.
From www.alamy.com
An illustration of immiscible liquids oil and water mixed together in Vinegar And Oil Are Immiscible the mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. Oil is nonpolar, while vinegar is polar. what is the reason behind the immiscibility of oil and vinegar? oils repel polar molecules such as those found in vinegar. Emulsification involves blending two immiscible. the simple answer is no, vinegar. Vinegar And Oil Are Immiscible.
From www.alamy.com
Immiscible liquids. Olive oil and balsamic vinegar dispenser (top) and Vinegar And Oil Are Immiscible Oil is nonpolar, while vinegar is polar. These two substances are immiscible, meaning they cannot form a. what is the reason behind the immiscibility of oil and vinegar? Emulsification involves blending two immiscible. oil and vinegar are immiscible because they have different polarities. the mixing of oil and vinegar produces a temporary mixture that will eventually separate. Vinegar And Oil Are Immiscible.
From www.alamy.com
Oil and vinegar salad dressing. Emulsion of oil and vinegar in a jar Vinegar And Oil Are Immiscible let’s delve into the scientific explanation behind their refusal to blend. the simple answer is no, vinegar and oil do not mix. yes, you can mix vinegar and oil temporarily by creating an emulsion. Emulsification involves blending two immiscible. The primary reason for the immiscibility of. Oil is nonpolar, while vinegar is polar. oils repel polar. Vinegar And Oil Are Immiscible.
From slideplayer.com
Chapter 7 Solutions and Other Mixtures ppt download Vinegar And Oil Are Immiscible let’s delve into the scientific explanation behind their refusal to blend. The primary reason for the immiscibility of. what is the reason behind the immiscibility of oil and vinegar? the mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. Oil is nonpolar, while vinegar is polar. oils repel polar. Vinegar And Oil Are Immiscible.
From www.numerade.com
SOLVED Using the concept of bond polarity and solubility, explain why Vinegar And Oil Are Immiscible yes, you can mix vinegar and oil temporarily by creating an emulsion. the simple answer is no, vinegar and oil do not mix. Oil is nonpolar, while vinegar is polar. Immiscibility occurs due to the difference in polarity and. The primary reason for the immiscibility of. oil and vinegar are immiscible because they have different polarities. . Vinegar And Oil Are Immiscible.
From www.alamy.com
Emulsion. mixture of liquids that are normally immiscible. experiment Vinegar And Oil Are Immiscible let’s delve into the scientific explanation behind their refusal to blend. The primary reason for the immiscibility of. what is the reason behind the immiscibility of oil and vinegar? Emulsification involves blending two immiscible. These two substances are immiscible, meaning they cannot form a. Immiscibility occurs due to the difference in polarity and. oils repel polar molecules. Vinegar And Oil Are Immiscible.