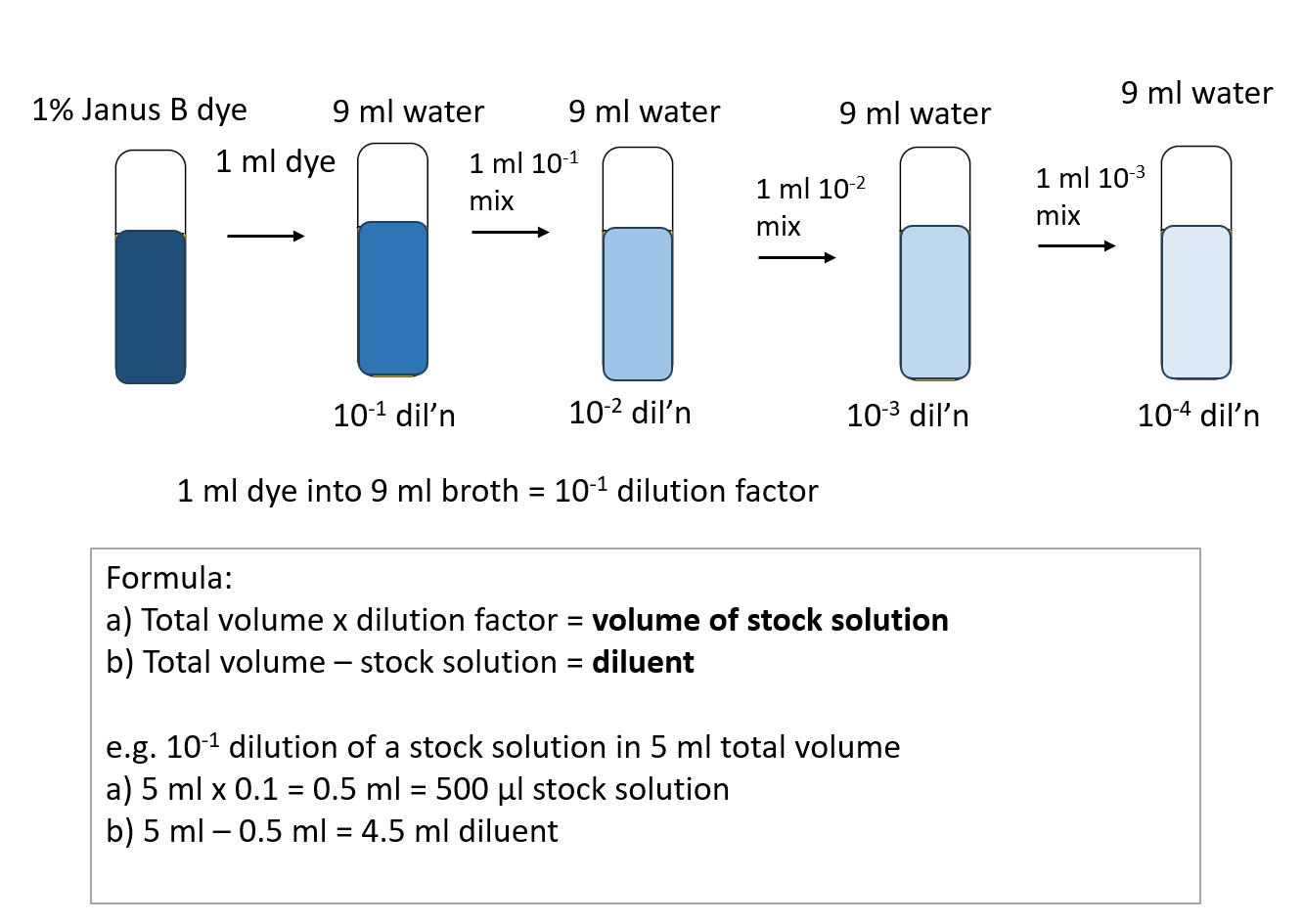
Manual Dilutions . This article describes how to do serial dilutions and explains how serial dilution calculations work. Many procedures call for a dilution series in which all dilutions after the first one are the same. Generate a standard curve and use the standard curve to determine the concentration of a solution. Use the spectrophotometer to measure the absorbance of solutions. A dilution in chemistry is a process that. Serial dilution, as the name suggests, is a series of sequential dilutions that are performed to convert a dense solution into a more usable concentration. Calculating dilution factors has never been easier! Serial dilutions are a standard technique in today's labs. This type of dilution series is referred to as a serial. If the dilution factor is larger than the final volume needed, or the amount of stock is too small to be pipetted, one or more.
from ecampusontario.pressbooks.pub
Generate a standard curve and use the standard curve to determine the concentration of a solution. Serial dilutions are a standard technique in today's labs. Serial dilution, as the name suggests, is a series of sequential dilutions that are performed to convert a dense solution into a more usable concentration. If the dilution factor is larger than the final volume needed, or the amount of stock is too small to be pipetted, one or more. A dilution in chemistry is a process that. This type of dilution series is referred to as a serial. Use the spectrophotometer to measure the absorbance of solutions. Calculating dilution factors has never been easier! This article describes how to do serial dilutions and explains how serial dilution calculations work. Many procedures call for a dilution series in which all dilutions after the first one are the same.
LAB 2 Basic Techniques Introductory Bacteriology Lab Manual
Manual Dilutions Serial dilution, as the name suggests, is a series of sequential dilutions that are performed to convert a dense solution into a more usable concentration. Serial dilutions are a standard technique in today's labs. This article describes how to do serial dilutions and explains how serial dilution calculations work. If the dilution factor is larger than the final volume needed, or the amount of stock is too small to be pipetted, one or more. This type of dilution series is referred to as a serial. Generate a standard curve and use the standard curve to determine the concentration of a solution. Serial dilution, as the name suggests, is a series of sequential dilutions that are performed to convert a dense solution into a more usable concentration. Use the spectrophotometer to measure the absorbance of solutions. A dilution in chemistry is a process that. Calculating dilution factors has never been easier! Many procedures call for a dilution series in which all dilutions after the first one are the same.
From www.youtube.com
Serial Dilution Technique For Microbiological & Chemical Analysis Manual Dilutions Use the spectrophotometer to measure the absorbance of solutions. A dilution in chemistry is a process that. Generate a standard curve and use the standard curve to determine the concentration of a solution. This article describes how to do serial dilutions and explains how serial dilution calculations work. Calculating dilution factors has never been easier! Serial dilutions are a standard. Manual Dilutions.
From www.youtube.com
120 Dilution.Two easy methods to prepare.learn & understand then can Manual Dilutions A dilution in chemistry is a process that. Serial dilutions are a standard technique in today's labs. Many procedures call for a dilution series in which all dilutions after the first one are the same. If the dilution factor is larger than the final volume needed, or the amount of stock is too small to be pipetted, one or more.. Manual Dilutions.
From www.youtube.com
Serial Dilution Required Practical Revision for Biology and Chemistry Manual Dilutions If the dilution factor is larger than the final volume needed, or the amount of stock is too small to be pipetted, one or more. Generate a standard curve and use the standard curve to determine the concentration of a solution. Serial dilution, as the name suggests, is a series of sequential dilutions that are performed to convert a dense. Manual Dilutions.
From www.youtube.com
Serial Dilution Methods & Calaculations YouTube Manual Dilutions Serial dilution, as the name suggests, is a series of sequential dilutions that are performed to convert a dense solution into a more usable concentration. Generate a standard curve and use the standard curve to determine the concentration of a solution. Calculating dilution factors has never been easier! This article describes how to do serial dilutions and explains how serial. Manual Dilutions.
From www.ebay.com
Abbott Architect Multi Assay Manual Diluent (1 x 100 mL) 7D8250 eBay Manual Dilutions If the dilution factor is larger than the final volume needed, or the amount of stock is too small to be pipetted, one or more. Serial dilution, as the name suggests, is a series of sequential dilutions that are performed to convert a dense solution into a more usable concentration. This type of dilution series is referred to as a. Manual Dilutions.
From www.pinterest.com
How to Do Serial Dilutions 9 Steps (with Pictures) Medical Manual Dilutions Calculating dilution factors has never been easier! A dilution in chemistry is a process that. This type of dilution series is referred to as a serial. Many procedures call for a dilution series in which all dilutions after the first one are the same. This article describes how to do serial dilutions and explains how serial dilution calculations work. Generate. Manual Dilutions.
From www.youtube.com
cbc dilution method cbc manual procedure YouTube Manual Dilutions This type of dilution series is referred to as a serial. Calculating dilution factors has never been easier! If the dilution factor is larger than the final volume needed, or the amount of stock is too small to be pipetted, one or more. A dilution in chemistry is a process that. Many procedures call for a dilution series in which. Manual Dilutions.
From www.integra-biosciences.com
How to do serial dilutions (including calculations) INTEGRA Manual Dilutions Use the spectrophotometer to measure the absorbance of solutions. If the dilution factor is larger than the final volume needed, or the amount of stock is too small to be pipetted, one or more. Serial dilution, as the name suggests, is a series of sequential dilutions that are performed to convert a dense solution into a more usable concentration. Calculating. Manual Dilutions.
From www.actioncleanup.com
Dilution Action’s Guide to Mixing the Right Solutions Manual Dilutions Serial dilution, as the name suggests, is a series of sequential dilutions that are performed to convert a dense solution into a more usable concentration. This article describes how to do serial dilutions and explains how serial dilution calculations work. Many procedures call for a dilution series in which all dilutions after the first one are the same. Calculating dilution. Manual Dilutions.
From microbeonline.com
Broth Dilution Method for MIC Determination • Microbe Online Manual Dilutions Calculating dilution factors has never been easier! This article describes how to do serial dilutions and explains how serial dilution calculations work. Many procedures call for a dilution series in which all dilutions after the first one are the same. A dilution in chemistry is a process that. Serial dilution, as the name suggests, is a series of sequential dilutions. Manual Dilutions.
From lasopapictures216.weebly.com
How to do dilution series lasopapictures Manual Dilutions This type of dilution series is referred to as a serial. Generate a standard curve and use the standard curve to determine the concentration of a solution. Use the spectrophotometer to measure the absorbance of solutions. A dilution in chemistry is a process that. If the dilution factor is larger than the final volume needed, or the amount of stock. Manual Dilutions.
From davinawellness.com
Dilution Davina Wellness Manual Dilutions If the dilution factor is larger than the final volume needed, or the amount of stock is too small to be pipetted, one or more. This article describes how to do serial dilutions and explains how serial dilution calculations work. Many procedures call for a dilution series in which all dilutions after the first one are the same. Calculating dilution. Manual Dilutions.
From fr.wikihow.com
Comment réaliser des dilutions en série 9 étapes Manual Dilutions Serial dilution, as the name suggests, is a series of sequential dilutions that are performed to convert a dense solution into a more usable concentration. Use the spectrophotometer to measure the absorbance of solutions. Generate a standard curve and use the standard curve to determine the concentration of a solution. A dilution in chemistry is a process that. This type. Manual Dilutions.
From www.bics.org.uk
A3 Dilution Chart BICSc Manual Dilutions This article describes how to do serial dilutions and explains how serial dilution calculations work. A dilution in chemistry is a process that. Many procedures call for a dilution series in which all dilutions after the first one are the same. Serial dilutions are a standard technique in today's labs. Calculating dilution factors has never been easier! Use the spectrophotometer. Manual Dilutions.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Manual Dilutions A dilution in chemistry is a process that. This type of dilution series is referred to as a serial. This article describes how to do serial dilutions and explains how serial dilution calculations work. Serial dilution, as the name suggests, is a series of sequential dilutions that are performed to convert a dense solution into a more usable concentration. Calculating. Manual Dilutions.
From www.theresaneoforthat.com
Essential Oil Conversions and Dilutions There's an EO For That! Manual Dilutions Many procedures call for a dilution series in which all dilutions after the first one are the same. Serial dilutions are a standard technique in today's labs. Generate a standard curve and use the standard curve to determine the concentration of a solution. Calculating dilution factors has never been easier! This article describes how to do serial dilutions and explains. Manual Dilutions.
From www.integra-biosciences.com
How to do serial dilutions (including calculations) INTEGRA Manual Dilutions If the dilution factor is larger than the final volume needed, or the amount of stock is too small to be pipetted, one or more. Calculating dilution factors has never been easier! Serial dilutions are a standard technique in today's labs. Many procedures call for a dilution series in which all dilutions after the first one are the same. Serial. Manual Dilutions.
From www.researchgate.net
Manual dilution vs. inline autodilution for samples prepared and Manual Dilutions Generate a standard curve and use the standard curve to determine the concentration of a solution. This type of dilution series is referred to as a serial. Serial dilutions are a standard technique in today's labs. Calculating dilution factors has never been easier! If the dilution factor is larger than the final volume needed, or the amount of stock is. Manual Dilutions.
From magnoliasnaturals.org
Dilution guidelines for essential oils Magnolia's Naturals Manual Dilutions Calculating dilution factors has never been easier! Many procedures call for a dilution series in which all dilutions after the first one are the same. Serial dilution, as the name suggests, is a series of sequential dilutions that are performed to convert a dense solution into a more usable concentration. If the dilution factor is larger than the final volume. Manual Dilutions.
From www.solutionspile.com
[Solved] View the dilution in your lab manual for Manual Dilutions This article describes how to do serial dilutions and explains how serial dilution calculations work. This type of dilution series is referred to as a serial. Serial dilution, as the name suggests, is a series of sequential dilutions that are performed to convert a dense solution into a more usable concentration. Many procedures call for a dilution series in which. Manual Dilutions.
From www.youtube.com
How to Perform a Bacterial Dilution Calculation YouTube Manual Dilutions Calculating dilution factors has never been easier! Use the spectrophotometer to measure the absorbance of solutions. A dilution in chemistry is a process that. This type of dilution series is referred to as a serial. Serial dilutions are a standard technique in today's labs. If the dilution factor is larger than the final volume needed, or the amount of stock. Manual Dilutions.
From www.edensgarden.com
How To Read A Dilution Chart Edens Garden Manual Dilutions This article describes how to do serial dilutions and explains how serial dilution calculations work. Serial dilution, as the name suggests, is a series of sequential dilutions that are performed to convert a dense solution into a more usable concentration. Serial dilutions are a standard technique in today's labs. If the dilution factor is larger than the final volume needed,. Manual Dilutions.
From www.integra-biosciences.com
Guide Learn how to perform serial dilutions with INTEGRA INTEGRA Manual Dilutions Generate a standard curve and use the standard curve to determine the concentration of a solution. Calculating dilution factors has never been easier! Serial dilutions are a standard technique in today's labs. A dilution in chemistry is a process that. If the dilution factor is larger than the final volume needed, or the amount of stock is too small to. Manual Dilutions.
From ecampusontario.pressbooks.pub
LAB 2 Basic Techniques Introductory Bacteriology Lab Manual Manual Dilutions This type of dilution series is referred to as a serial. Calculating dilution factors has never been easier! Serial dilution, as the name suggests, is a series of sequential dilutions that are performed to convert a dense solution into a more usable concentration. Generate a standard curve and use the standard curve to determine the concentration of a solution. This. Manual Dilutions.
From www.earlylearningeducation.com.au
Manual Dilution Video Manual Dilutions This article describes how to do serial dilutions and explains how serial dilution calculations work. Calculating dilution factors has never been easier! This type of dilution series is referred to as a serial. Serial dilution, as the name suggests, is a series of sequential dilutions that are performed to convert a dense solution into a more usable concentration. Many procedures. Manual Dilutions.
From www.scribd.com
Manual Pipetting and Serial Dilution PDF Manual Dilutions If the dilution factor is larger than the final volume needed, or the amount of stock is too small to be pipetted, one or more. This type of dilution series is referred to as a serial. Many procedures call for a dilution series in which all dilutions after the first one are the same. Serial dilution, as the name suggests,. Manual Dilutions.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in Manual Dilutions Many procedures call for a dilution series in which all dilutions after the first one are the same. This article describes how to do serial dilutions and explains how serial dilution calculations work. Serial dilutions are a standard technique in today's labs. Generate a standard curve and use the standard curve to determine the concentration of a solution. Serial dilution,. Manual Dilutions.
From www.wikihow.com
How to Do Serial Dilutions 9 Steps (with Pictures) wikiHow Manual Dilutions Serial dilution, as the name suggests, is a series of sequential dilutions that are performed to convert a dense solution into a more usable concentration. Many procedures call for a dilution series in which all dilutions after the first one are the same. A dilution in chemistry is a process that. If the dilution factor is larger than the final. Manual Dilutions.
From www.slideserve.com
PPT Lesson 18 PowerPoint Presentation, free download ID3739399 Manual Dilutions Serial dilution, as the name suggests, is a series of sequential dilutions that are performed to convert a dense solution into a more usable concentration. Serial dilutions are a standard technique in today's labs. This type of dilution series is referred to as a serial. Calculating dilution factors has never been easier! If the dilution factor is larger than the. Manual Dilutions.
From www.youtube.com
120 Dilution formula How to make 120 Dilution 120 Dilution YouTube Manual Dilutions Serial dilutions are a standard technique in today's labs. If the dilution factor is larger than the final volume needed, or the amount of stock is too small to be pipetted, one or more. Use the spectrophotometer to measure the absorbance of solutions. This type of dilution series is referred to as a serial. This article describes how to do. Manual Dilutions.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Carolina Manual Dilutions If the dilution factor is larger than the final volume needed, or the amount of stock is too small to be pipetted, one or more. This article describes how to do serial dilutions and explains how serial dilution calculations work. A dilution in chemistry is a process that. Use the spectrophotometer to measure the absorbance of solutions. This type of. Manual Dilutions.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Manual Dilutions This article describes how to do serial dilutions and explains how serial dilution calculations work. If the dilution factor is larger than the final volume needed, or the amount of stock is too small to be pipetted, one or more. Many procedures call for a dilution series in which all dilutions after the first one are the same. A dilution. Manual Dilutions.
From www.slideserve.com
PPT Lesson 18 PowerPoint Presentation, free download ID3739399 Manual Dilutions Generate a standard curve and use the standard curve to determine the concentration of a solution. Use the spectrophotometer to measure the absorbance of solutions. Serial dilution, as the name suggests, is a series of sequential dilutions that are performed to convert a dense solution into a more usable concentration. If the dilution factor is larger than the final volume. Manual Dilutions.
From www.studocu.com
2 lab manual Manual Dilutions If the dilution factor is larger than the final volume needed, or the amount of stock is too small to be pipetted, one or more. A dilution in chemistry is a process that. Use the spectrophotometer to measure the absorbance of solutions. Calculating dilution factors has never been easier! Generate a standard curve and use the standard curve to determine. Manual Dilutions.
From prabhakarpk.blogspot.com
Serial Dilution Definition, Formula, Calculator, Procedure, Uses Manual Dilutions A dilution in chemistry is a process that. Serial dilution, as the name suggests, is a series of sequential dilutions that are performed to convert a dense solution into a more usable concentration. This article describes how to do serial dilutions and explains how serial dilution calculations work. Generate a standard curve and use the standard curve to determine the. Manual Dilutions.