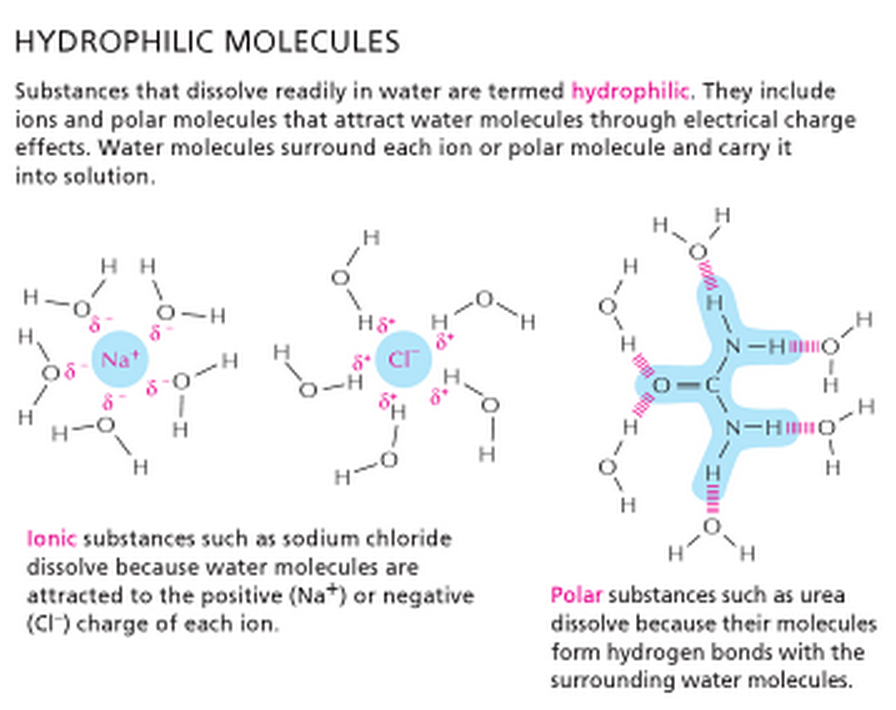
What Causes A Compound To Be Hydrophilic . Materials with a special affinity for water — those it spreads across, maximizing contact — are known as hydrophilic. Water itself is a polar molecule, made up of one. The terms hydrophilic and hydrophobic are used to describe molecules or substances based on how they react to water molecules. Those that naturally repel water, causing droplets to form, are known as hydrophobic. Hydrophobic and hydrophilic forces are interactions that serve to keep chemical groups positioned close to one another. The degree or extent to which a molecule or surface attracts water is known as the ‘ hydrophilicity ‘ of that molecule. Molecules are hydrophilic when they have charges or partial charges. They are typically polar and capable of forming hydrogen bonds with water,. These types of molecules are called polar. Some of the most common examples of hydrophilic substances are sugar, salt, starch, and cellulose. Hydrophilic substances are those that have an affinity for water.
from joannelovesscience.com
Those that naturally repel water, causing droplets to form, are known as hydrophobic. Hydrophobic and hydrophilic forces are interactions that serve to keep chemical groups positioned close to one another. The terms hydrophilic and hydrophobic are used to describe molecules or substances based on how they react to water molecules. Some of the most common examples of hydrophilic substances are sugar, salt, starch, and cellulose. Water itself is a polar molecule, made up of one. The degree or extent to which a molecule or surface attracts water is known as the ‘ hydrophilicity ‘ of that molecule. They are typically polar and capable of forming hydrogen bonds with water,. Hydrophilic substances are those that have an affinity for water. Molecules are hydrophilic when they have charges or partial charges. These types of molecules are called polar.
Chemistry of Mascara Joanne Loves Science
What Causes A Compound To Be Hydrophilic The degree or extent to which a molecule or surface attracts water is known as the ‘ hydrophilicity ‘ of that molecule. Hydrophilic substances are those that have an affinity for water. These types of molecules are called polar. Some of the most common examples of hydrophilic substances are sugar, salt, starch, and cellulose. Hydrophobic and hydrophilic forces are interactions that serve to keep chemical groups positioned close to one another. The terms hydrophilic and hydrophobic are used to describe molecules or substances based on how they react to water molecules. The degree or extent to which a molecule or surface attracts water is known as the ‘ hydrophilicity ‘ of that molecule. Molecules are hydrophilic when they have charges or partial charges. Materials with a special affinity for water — those it spreads across, maximizing contact — are known as hydrophilic. Those that naturally repel water, causing droplets to form, are known as hydrophobic. Water itself is a polar molecule, made up of one. They are typically polar and capable of forming hydrogen bonds with water,.
From slideplayer.com
Organic Molecules Unit 3 Lesson ppt download What Causes A Compound To Be Hydrophilic Materials with a special affinity for water — those it spreads across, maximizing contact — are known as hydrophilic. Hydrophobic and hydrophilic forces are interactions that serve to keep chemical groups positioned close to one another. The terms hydrophilic and hydrophobic are used to describe molecules or substances based on how they react to water molecules. These types of molecules. What Causes A Compound To Be Hydrophilic.
From testbook.com
Learn Difference Between Hydrophobic and Hydrophilic Amino Acids What Causes A Compound To Be Hydrophilic Materials with a special affinity for water — those it spreads across, maximizing contact — are known as hydrophilic. Water itself is a polar molecule, made up of one. Some of the most common examples of hydrophilic substances are sugar, salt, starch, and cellulose. Those that naturally repel water, causing droplets to form, are known as hydrophobic. Hydrophobic and hydrophilic. What Causes A Compound To Be Hydrophilic.
From aminoco.com
What Are Hydrophilic Amino Acids? The Amino Company What Causes A Compound To Be Hydrophilic They are typically polar and capable of forming hydrogen bonds with water,. Some of the most common examples of hydrophilic substances are sugar, salt, starch, and cellulose. Water itself is a polar molecule, made up of one. Molecules are hydrophilic when they have charges or partial charges. Hydrophobic and hydrophilic forces are interactions that serve to keep chemical groups positioned. What Causes A Compound To Be Hydrophilic.
From depositphotos.com
Hydrophobic vs hydrophilic surface effect on water drop outline diagram What Causes A Compound To Be Hydrophilic Molecules are hydrophilic when they have charges or partial charges. Those that naturally repel water, causing droplets to form, are known as hydrophobic. Hydrophobic and hydrophilic forces are interactions that serve to keep chemical groups positioned close to one another. Water itself is a polar molecule, made up of one. They are typically polar and capable of forming hydrogen bonds. What Causes A Compound To Be Hydrophilic.
From chemistrylearninghub.com
Understanding the Hydrophilic Effect Unveiling the Meaning behind the What Causes A Compound To Be Hydrophilic They are typically polar and capable of forming hydrogen bonds with water,. Materials with a special affinity for water — those it spreads across, maximizing contact — are known as hydrophilic. The terms hydrophilic and hydrophobic are used to describe molecules or substances based on how they react to water molecules. The degree or extent to which a molecule or. What Causes A Compound To Be Hydrophilic.
From www.chegg.com
Solved Is this compound hydrophilic? Yes No Depending on the What Causes A Compound To Be Hydrophilic Some of the most common examples of hydrophilic substances are sugar, salt, starch, and cellulose. These types of molecules are called polar. Hydrophilic substances are those that have an affinity for water. Molecules are hydrophilic when they have charges or partial charges. Water itself is a polar molecule, made up of one. They are typically polar and capable of forming. What Causes A Compound To Be Hydrophilic.
From slideplayer.com
Nanotechnology. ppt download What Causes A Compound To Be Hydrophilic The degree or extent to which a molecule or surface attracts water is known as the ‘ hydrophilicity ‘ of that molecule. Those that naturally repel water, causing droplets to form, are known as hydrophobic. Some of the most common examples of hydrophilic substances are sugar, salt, starch, and cellulose. These types of molecules are called polar. Water itself is. What Causes A Compound To Be Hydrophilic.
From www.chegg.com
Solved Which of the following compounds is most hydrophilic? What Causes A Compound To Be Hydrophilic The terms hydrophilic and hydrophobic are used to describe molecules or substances based on how they react to water molecules. Those that naturally repel water, causing droplets to form, are known as hydrophobic. Some of the most common examples of hydrophilic substances are sugar, salt, starch, and cellulose. Hydrophobic and hydrophilic forces are interactions that serve to keep chemical groups. What Causes A Compound To Be Hydrophilic.
From www.differencebetween.com
Difference Between Hydrophilic and Hydrophobic Compare the Difference What Causes A Compound To Be Hydrophilic These types of molecules are called polar. Those that naturally repel water, causing droplets to form, are known as hydrophobic. Hydrophobic and hydrophilic forces are interactions that serve to keep chemical groups positioned close to one another. The degree or extent to which a molecule or surface attracts water is known as the ‘ hydrophilicity ‘ of that molecule. The. What Causes A Compound To Be Hydrophilic.
From childhealthpolicy.vumc.org
🏆 Hydrophobic and hydrophilic interactions in proteins. How do What Causes A Compound To Be Hydrophilic The terms hydrophilic and hydrophobic are used to describe molecules or substances based on how they react to water molecules. Water itself is a polar molecule, made up of one. These types of molecules are called polar. Molecules are hydrophilic when they have charges or partial charges. Some of the most common examples of hydrophilic substances are sugar, salt, starch,. What Causes A Compound To Be Hydrophilic.
From www.chegg.com
Solved Is the molecule, shown below hydrophilic, What Causes A Compound To Be Hydrophilic The terms hydrophilic and hydrophobic are used to describe molecules or substances based on how they react to water molecules. Some of the most common examples of hydrophilic substances are sugar, salt, starch, and cellulose. Materials with a special affinity for water — those it spreads across, maximizing contact — are known as hydrophilic. Those that naturally repel water, causing. What Causes A Compound To Be Hydrophilic.
From www.youtube.com
Hydrophilic vs. Hydrophobic YouTube What Causes A Compound To Be Hydrophilic Some of the most common examples of hydrophilic substances are sugar, salt, starch, and cellulose. These types of molecules are called polar. Water itself is a polar molecule, made up of one. The terms hydrophilic and hydrophobic are used to describe molecules or substances based on how they react to water molecules. The degree or extent to which a molecule. What Causes A Compound To Be Hydrophilic.
From slidetodoc.com
Solubility and Net Ionic Equations Focus on equations What Causes A Compound To Be Hydrophilic They are typically polar and capable of forming hydrogen bonds with water,. The terms hydrophilic and hydrophobic are used to describe molecules or substances based on how they react to water molecules. Hydrophilic substances are those that have an affinity for water. Molecules are hydrophilic when they have charges or partial charges. Some of the most common examples of hydrophilic. What Causes A Compound To Be Hydrophilic.
From www.studocu.com
BIOL 1345 Module 2 Water Hydrophilic and Hydrophobic Substances A What Causes A Compound To Be Hydrophilic Hydrophilic substances are those that have an affinity for water. Some of the most common examples of hydrophilic substances are sugar, salt, starch, and cellulose. These types of molecules are called polar. The terms hydrophilic and hydrophobic are used to describe molecules or substances based on how they react to water molecules. Those that naturally repel water, causing droplets to. What Causes A Compound To Be Hydrophilic.
From www.slideshare.net
Molecular Biology 14 What Causes A Compound To Be Hydrophilic Some of the most common examples of hydrophilic substances are sugar, salt, starch, and cellulose. The terms hydrophilic and hydrophobic are used to describe molecules or substances based on how they react to water molecules. Materials with a special affinity for water — those it spreads across, maximizing contact — are known as hydrophilic. Hydrophilic substances are those that have. What Causes A Compound To Be Hydrophilic.
From www.slideserve.com
PPT Chapter 3. Water— The Elixir of Life! PowerPoint Presentation What Causes A Compound To Be Hydrophilic The terms hydrophilic and hydrophobic are used to describe molecules or substances based on how they react to water molecules. Materials with a special affinity for water — those it spreads across, maximizing contact — are known as hydrophilic. They are typically polar and capable of forming hydrogen bonds with water,. Those that naturally repel water, causing droplets to form,. What Causes A Compound To Be Hydrophilic.
From www.researchgate.net
Steps to encapsulate hydrophilic antioxidants into alginatewhey What Causes A Compound To Be Hydrophilic The degree or extent to which a molecule or surface attracts water is known as the ‘ hydrophilicity ‘ of that molecule. Water itself is a polar molecule, made up of one. The terms hydrophilic and hydrophobic are used to describe molecules or substances based on how they react to water molecules. Hydrophilic substances are those that have an affinity. What Causes A Compound To Be Hydrophilic.
From www.pinterest.at
Image result for hydrophobic molecules Molecules, Hydrophobic, Ap chem What Causes A Compound To Be Hydrophilic Molecules are hydrophilic when they have charges or partial charges. The terms hydrophilic and hydrophobic are used to describe molecules or substances based on how they react to water molecules. The degree or extent to which a molecule or surface attracts water is known as the ‘ hydrophilicity ‘ of that molecule. Hydrophilic substances are those that have an affinity. What Causes A Compound To Be Hydrophilic.
From slideplayer.com
Topic 2.1 Molecules to metabolism ppt download What Causes A Compound To Be Hydrophilic Molecules are hydrophilic when they have charges or partial charges. These types of molecules are called polar. Hydrophilic substances are those that have an affinity for water. Some of the most common examples of hydrophilic substances are sugar, salt, starch, and cellulose. Those that naturally repel water, causing droplets to form, are known as hydrophobic. Hydrophobic and hydrophilic forces are. What Causes A Compound To Be Hydrophilic.
From slideplayer.com
Introduction to Chemistry ppt download What Causes A Compound To Be Hydrophilic The terms hydrophilic and hydrophobic are used to describe molecules or substances based on how they react to water molecules. Hydrophilic substances are those that have an affinity for water. They are typically polar and capable of forming hydrogen bonds with water,. Those that naturally repel water, causing droplets to form, are known as hydrophobic. Molecules are hydrophilic when they. What Causes A Compound To Be Hydrophilic.
From courses.lumenlearning.com
Components and Structure OpenStax Biology 2e What Causes A Compound To Be Hydrophilic Water itself is a polar molecule, made up of one. Materials with a special affinity for water — those it spreads across, maximizing contact — are known as hydrophilic. Those that naturally repel water, causing droplets to form, are known as hydrophobic. Hydrophobic and hydrophilic forces are interactions that serve to keep chemical groups positioned close to one another. The. What Causes A Compound To Be Hydrophilic.
From www.slideshare.net
Phospholipids § Hydrophobic or hydrophilic? What Causes A Compound To Be Hydrophilic These types of molecules are called polar. Water itself is a polar molecule, made up of one. Hydrophilic substances are those that have an affinity for water. Some of the most common examples of hydrophilic substances are sugar, salt, starch, and cellulose. Hydrophobic and hydrophilic forces are interactions that serve to keep chemical groups positioned close to one another. Those. What Causes A Compound To Be Hydrophilic.
From www.numerade.com
SOLVEDDescribe how hydrophilic and hydrophobic colloids are stabilized What Causes A Compound To Be Hydrophilic Hydrophilic substances are those that have an affinity for water. Materials with a special affinity for water — those it spreads across, maximizing contact — are known as hydrophilic. They are typically polar and capable of forming hydrogen bonds with water,. Hydrophobic and hydrophilic forces are interactions that serve to keep chemical groups positioned close to one another. These types. What Causes A Compound To Be Hydrophilic.
From slideplayer.com
Chapter 3 The Molecules of Cells Lecture by Richard L. Myers ppt download What Causes A Compound To Be Hydrophilic Molecules are hydrophilic when they have charges or partial charges. They are typically polar and capable of forming hydrogen bonds with water,. These types of molecules are called polar. The degree or extent to which a molecule or surface attracts water is known as the ‘ hydrophilicity ‘ of that molecule. Some of the most common examples of hydrophilic substances. What Causes A Compound To Be Hydrophilic.
From slideplayer.com
NOTES 2.2 Properties of Water ppt download What Causes A Compound To Be Hydrophilic Molecules are hydrophilic when they have charges or partial charges. Water itself is a polar molecule, made up of one. Hydrophobic and hydrophilic forces are interactions that serve to keep chemical groups positioned close to one another. Materials with a special affinity for water — those it spreads across, maximizing contact — are known as hydrophilic. Hydrophilic substances are those. What Causes A Compound To Be Hydrophilic.
From exokuwuse.blob.core.windows.net
What Makes A Compound Hydrophobic And Hydrophilic at Betty Gable blog What Causes A Compound To Be Hydrophilic Water itself is a polar molecule, made up of one. Materials with a special affinity for water — those it spreads across, maximizing contact — are known as hydrophilic. Hydrophilic substances are those that have an affinity for water. Those that naturally repel water, causing droplets to form, are known as hydrophobic. Some of the most common examples of hydrophilic. What Causes A Compound To Be Hydrophilic.
From www.biologyonline.com
Hydrophilic Definition and Examples Biology Online Dictionary What Causes A Compound To Be Hydrophilic They are typically polar and capable of forming hydrogen bonds with water,. Hydrophobic and hydrophilic forces are interactions that serve to keep chemical groups positioned close to one another. Water itself is a polar molecule, made up of one. Hydrophilic substances are those that have an affinity for water. The terms hydrophilic and hydrophobic are used to describe molecules or. What Causes A Compound To Be Hydrophilic.
From joannelovesscience.com
Chemistry of Mascara Joanne Loves Science What Causes A Compound To Be Hydrophilic The degree or extent to which a molecule or surface attracts water is known as the ‘ hydrophilicity ‘ of that molecule. Those that naturally repel water, causing droplets to form, are known as hydrophobic. The terms hydrophilic and hydrophobic are used to describe molecules or substances based on how they react to water molecules. Water itself is a polar. What Causes A Compound To Be Hydrophilic.
From pediaa.com
Difference Between Hydrophobic and Hydrophilic Molecules Definition What Causes A Compound To Be Hydrophilic The degree or extent to which a molecule or surface attracts water is known as the ‘ hydrophilicity ‘ of that molecule. Hydrophobic and hydrophilic forces are interactions that serve to keep chemical groups positioned close to one another. Materials with a special affinity for water — those it spreads across, maximizing contact — are known as hydrophilic. They are. What Causes A Compound To Be Hydrophilic.
From www.numerade.com
SOLVED 5. The peptide bond is a resonance hybrid of two canonical What Causes A Compound To Be Hydrophilic Molecules are hydrophilic when they have charges or partial charges. The terms hydrophilic and hydrophobic are used to describe molecules or substances based on how they react to water molecules. Those that naturally repel water, causing droplets to form, are known as hydrophobic. Water itself is a polar molecule, made up of one. Materials with a special affinity for water. What Causes A Compound To Be Hydrophilic.
From www.slideserve.com
PPT 2.2 Water PowerPoint Presentation ID6361297 What Causes A Compound To Be Hydrophilic Hydrophilic substances are those that have an affinity for water. These types of molecules are called polar. Materials with a special affinity for water — those it spreads across, maximizing contact — are known as hydrophilic. Hydrophobic and hydrophilic forces are interactions that serve to keep chemical groups positioned close to one another. Those that naturally repel water, causing droplets. What Causes A Compound To Be Hydrophilic.
From www.youtube.com
Hydrophilic vs Hydrophobic Substances Cell Membranes YouTube What Causes A Compound To Be Hydrophilic They are typically polar and capable of forming hydrogen bonds with water,. The degree or extent to which a molecule or surface attracts water is known as the ‘ hydrophilicity ‘ of that molecule. Hydrophilic substances are those that have an affinity for water. Molecules are hydrophilic when they have charges or partial charges. The terms hydrophilic and hydrophobic are. What Causes A Compound To Be Hydrophilic.
From www.youtube.com
HYDROGEN BONDS (HYDROPHILIC VS HYDROPHOBIC) YouTube What Causes A Compound To Be Hydrophilic Those that naturally repel water, causing droplets to form, are known as hydrophobic. Some of the most common examples of hydrophilic substances are sugar, salt, starch, and cellulose. These types of molecules are called polar. Molecules are hydrophilic when they have charges or partial charges. Materials with a special affinity for water — those it spreads across, maximizing contact —. What Causes A Compound To Be Hydrophilic.
From www.slideserve.com
PPT Water and It’s properties PowerPoint Presentation, free download What Causes A Compound To Be Hydrophilic The degree or extent to which a molecule or surface attracts water is known as the ‘ hydrophilicity ‘ of that molecule. These types of molecules are called polar. Molecules are hydrophilic when they have charges or partial charges. Water itself is a polar molecule, made up of one. Some of the most common examples of hydrophilic substances are sugar,. What Causes A Compound To Be Hydrophilic.
From psiberg.com
Hydrophobic vs. Hydrophilic Molecules (Examples and Applications) What Causes A Compound To Be Hydrophilic The degree or extent to which a molecule or surface attracts water is known as the ‘ hydrophilicity ‘ of that molecule. Water itself is a polar molecule, made up of one. Those that naturally repel water, causing droplets to form, are known as hydrophobic. These types of molecules are called polar. Materials with a special affinity for water —. What Causes A Compound To Be Hydrophilic.