Does Lead Have A Higher Heat Capacity Than Water . Lead 0.128 water has a particularly high specific heat compared to many other substances. How can the heat capacity of a lead sinker be determined? Specific heat capacity is the measurement of how much energy (in j). Because of its much larger mass,. Specific heat of lead is 0.13 j/g k. It is the thermal capacity per unit mass which is. The heat capacity of an object depends both on its mass and its chemical composition. The specific heat of metals are lower than that of water. How do the specific heats of metals compare with water? Lead is used because its specific thermal capacity is about 1/30 that of water. When a given amount of heat is added to different. The reason is that water has a greater specific heat than most common substances and thus undergoes a small temperature change for a. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of.
from www.youtube.com
The heat capacity of an object depends both on its mass and its chemical composition. How can the heat capacity of a lead sinker be determined? The reason is that water has a greater specific heat than most common substances and thus undergoes a small temperature change for a. How do the specific heats of metals compare with water? Specific heat capacity is the measurement of how much energy (in j). Lead 0.128 water has a particularly high specific heat compared to many other substances. Specific heat of lead is 0.13 j/g k. The specific heat of metals are lower than that of water. Because of its much larger mass,. It is the thermal capacity per unit mass which is.
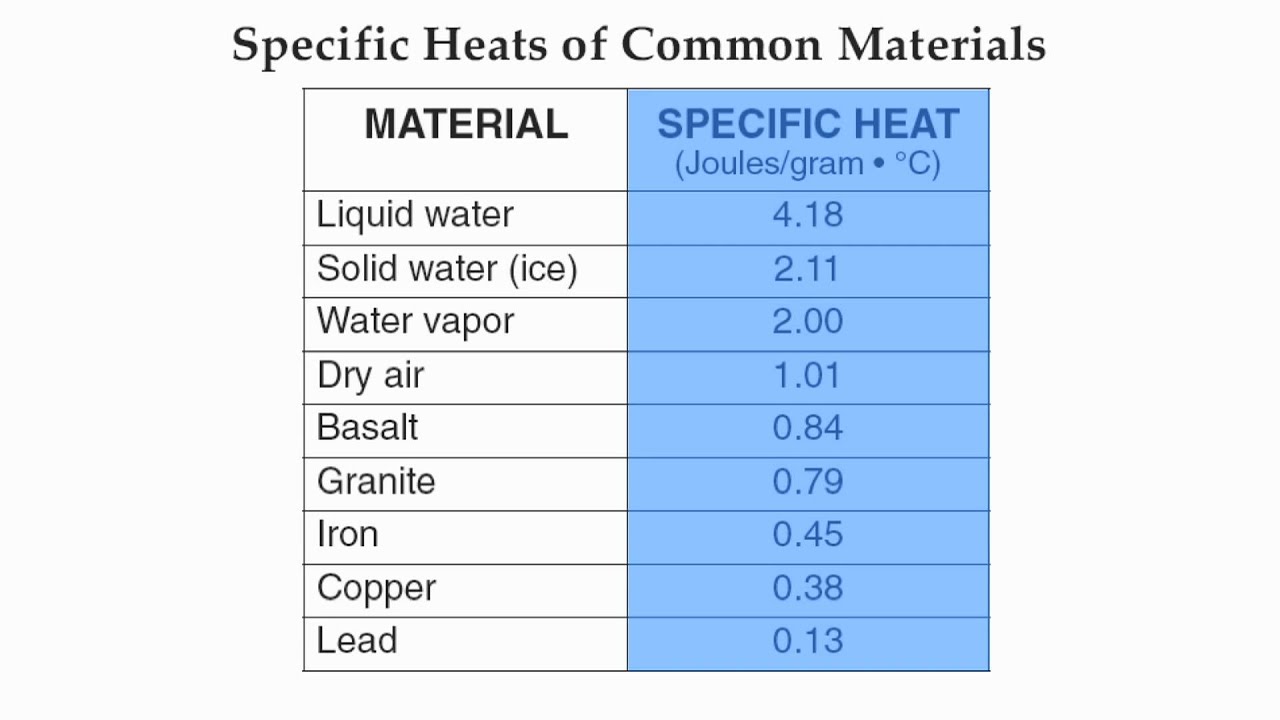
Reference Table Page 1Specific Heat of Common MaterialsHommocks Earth
Does Lead Have A Higher Heat Capacity Than Water The specific heat of metals are lower than that of water. Specific heat of lead is 0.13 j/g k. The heat capacity of an object depends both on its mass and its chemical composition. Because of its much larger mass,. The reason is that water has a greater specific heat than most common substances and thus undergoes a small temperature change for a. How do the specific heats of metals compare with water? How can the heat capacity of a lead sinker be determined? The specific heat of metals are lower than that of water. It is the thermal capacity per unit mass which is. When a given amount of heat is added to different. Lead 0.128 water has a particularly high specific heat compared to many other substances. Specific heat capacity is the measurement of how much energy (in j). Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Lead is used because its specific thermal capacity is about 1/30 that of water.
From www.slideserve.com
PPT Conceptual Physics 11 th Edition PowerPoint Presentation, free Does Lead Have A Higher Heat Capacity Than Water The specific heat of metals are lower than that of water. The heat capacity of an object depends both on its mass and its chemical composition. When a given amount of heat is added to different. Specific heat capacity is the measurement of how much energy (in j). Lead is used because its specific thermal capacity is about 1/30 that. Does Lead Have A Higher Heat Capacity Than Water.
From www.slideserve.com
PPT Chapter 10 Temperature and Heat PowerPoint Presentation, free Does Lead Have A Higher Heat Capacity Than Water Lead 0.128 water has a particularly high specific heat compared to many other substances. Specific heat of lead is 0.13 j/g k. The heat capacity of an object depends both on its mass and its chemical composition. Specific heat capacity is the measurement of how much energy (in j). The specific heat of metals are lower than that of water.. Does Lead Have A Higher Heat Capacity Than Water.
From lessonmagicziegler.z21.web.core.windows.net
Worksheet Introduction To Specific Heat Capacities Does Lead Have A Higher Heat Capacity Than Water The specific heat of metals are lower than that of water. How can the heat capacity of a lead sinker be determined? The reason is that water has a greater specific heat than most common substances and thus undergoes a small temperature change for a. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Because. Does Lead Have A Higher Heat Capacity Than Water.
From www.slideserve.com
PPT Chapter 10 Temperature and Heat PowerPoint Presentation, free Does Lead Have A Higher Heat Capacity Than Water The specific heat of metals are lower than that of water. The reason is that water has a greater specific heat than most common substances and thus undergoes a small temperature change for a. How can the heat capacity of a lead sinker be determined? Lead 0.128 water has a particularly high specific heat compared to many other substances. Because. Does Lead Have A Higher Heat Capacity Than Water.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Does Lead Have A Higher Heat Capacity Than Water Lead 0.128 water has a particularly high specific heat compared to many other substances. The heat capacity of an object depends both on its mass and its chemical composition. Because of its much larger mass,. It is the thermal capacity per unit mass which is. Lead is used because its specific thermal capacity is about 1/30 that of water. Specific. Does Lead Have A Higher Heat Capacity Than Water.
From www.chegg.com
Solved explain why heat capacity for lead and other metals Does Lead Have A Higher Heat Capacity Than Water When a given amount of heat is added to different. The specific heat of metals are lower than that of water. How do the specific heats of metals compare with water? Specific heat of lead is 0.13 j/g k. The reason is that water has a greater specific heat than most common substances and thus undergoes a small temperature change. Does Lead Have A Higher Heat Capacity Than Water.
From www.animalia-life.club
Specific Heat Chart Of Common Substances Does Lead Have A Higher Heat Capacity Than Water The heat capacity of an object depends both on its mass and its chemical composition. How can the heat capacity of a lead sinker be determined? Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Specific heat of lead is 0.13 j/g k. The specific heat of metals are lower than that of water. Lead. Does Lead Have A Higher Heat Capacity Than Water.
From www.slideserve.com
PPT Water’s Influence on Weather & Climate PowerPoint Presentation Does Lead Have A Higher Heat Capacity Than Water Specific heat of lead is 0.13 j/g k. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. It is the thermal capacity per unit mass which is. The specific heat of metals are lower than that of water. The heat capacity of an object depends both on its mass and its chemical composition. How can. Does Lead Have A Higher Heat Capacity Than Water.
From www.showme.com
Properties of water high heat capacity Science ShowMe Does Lead Have A Higher Heat Capacity Than Water The specific heat of metals are lower than that of water. It is the thermal capacity per unit mass which is. When a given amount of heat is added to different. How do the specific heats of metals compare with water? How can the heat capacity of a lead sinker be determined? Because of its much larger mass,. Specific heat. Does Lead Have A Higher Heat Capacity Than Water.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Does Lead Have A Higher Heat Capacity Than Water Because of its much larger mass,. Lead is used because its specific thermal capacity is about 1/30 that of water. Specific heat of lead is 0.13 j/g k. How can the heat capacity of a lead sinker be determined? Specific heat capacity is the measurement of how much energy (in j). It is the thermal capacity per unit mass which. Does Lead Have A Higher Heat Capacity Than Water.
From slideplayer.com
Properties of Water Polar covalent bond unequal sharing of electrons Does Lead Have A Higher Heat Capacity Than Water The heat capacity of an object depends both on its mass and its chemical composition. It is the thermal capacity per unit mass which is. How can the heat capacity of a lead sinker be determined? Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Lead 0.128 water has a particularly high specific heat compared. Does Lead Have A Higher Heat Capacity Than Water.
From www.careerpower.in
What is Heat Capacity Definition, Equations, Examples and Types Does Lead Have A Higher Heat Capacity Than Water How can the heat capacity of a lead sinker be determined? Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. How do the specific heats of metals compare with water? The reason is that water has a greater specific heat than most common substances and thus undergoes a small temperature change for a. Specific heat. Does Lead Have A Higher Heat Capacity Than Water.
From en.ppt-online.org
Class water online presentation Does Lead Have A Higher Heat Capacity Than Water How do the specific heats of metals compare with water? It is the thermal capacity per unit mass which is. Specific heat capacity is the measurement of how much energy (in j). The specific heat of metals are lower than that of water. Because of its much larger mass,. The heat capacity of an object depends both on its mass. Does Lead Have A Higher Heat Capacity Than Water.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID6095414 Does Lead Have A Higher Heat Capacity Than Water Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Lead is used because its specific thermal capacity is about 1/30 that of water. Specific heat capacity is the measurement of how much energy (in j). Because of its much larger mass,. How do the specific heats of metals compare with water? When a given amount. Does Lead Have A Higher Heat Capacity Than Water.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii Does Lead Have A Higher Heat Capacity Than Water Specific heat capacity is the measurement of how much energy (in j). Because of its much larger mass,. How can the heat capacity of a lead sinker be determined? When a given amount of heat is added to different. The specific heat of metals are lower than that of water. Specific heat of lead is 0.13 j/g k. How do. Does Lead Have A Higher Heat Capacity Than Water.
From slideplayer.com
PROPERTIES OF SEAWATER ppt download Does Lead Have A Higher Heat Capacity Than Water The specific heat of metals are lower than that of water. The reason is that water has a greater specific heat than most common substances and thus undergoes a small temperature change for a. Lead 0.128 water has a particularly high specific heat compared to many other substances. Specific heat of lead is 0.13 j/g k. It is the thermal. Does Lead Have A Higher Heat Capacity Than Water.
From support.rollsbattery.com
Temperature vs. Capacity Flooded Lead Acid Batteries Technical Support Does Lead Have A Higher Heat Capacity Than Water The heat capacity of an object depends both on its mass and its chemical composition. Because of its much larger mass,. How can the heat capacity of a lead sinker be determined? The reason is that water has a greater specific heat than most common substances and thus undergoes a small temperature change for a. Lead 0.128 water has a. Does Lead Have A Higher Heat Capacity Than Water.
From www.animalia-life.club
Heat Capacity Of Water Does Lead Have A Higher Heat Capacity Than Water The heat capacity of an object depends both on its mass and its chemical composition. Specific heat of lead is 0.13 j/g k. Lead 0.128 water has a particularly high specific heat compared to many other substances. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. The specific heat of metals are lower than that. Does Lead Have A Higher Heat Capacity Than Water.
From www.slideserve.com
PPT SPECIFIC HEAT CAPACITY PowerPoint Presentation, free download Does Lead Have A Higher Heat Capacity Than Water How can the heat capacity of a lead sinker be determined? The reason is that water has a greater specific heat than most common substances and thus undergoes a small temperature change for a. It is the thermal capacity per unit mass which is. Lead 0.128 water has a particularly high specific heat compared to many other substances. Specific heat. Does Lead Have A Higher Heat Capacity Than Water.
From www.geeksforgeeks.org
Heat Capacity Does Lead Have A Higher Heat Capacity Than Water Specific heat of lead is 0.13 j/g k. Because of its much larger mass,. Lead is used because its specific thermal capacity is about 1/30 that of water. Lead 0.128 water has a particularly high specific heat compared to many other substances. How can the heat capacity of a lead sinker be determined? Specific heat capacity is the measurement of. Does Lead Have A Higher Heat Capacity Than Water.
From www.chegg.com
Solved Water has a higher specific heat capacity than lead Does Lead Have A Higher Heat Capacity Than Water The specific heat of metals are lower than that of water. The reason is that water has a greater specific heat than most common substances and thus undergoes a small temperature change for a. Specific heat capacity is the measurement of how much energy (in j). Lead 0.128 water has a particularly high specific heat compared to many other substances.. Does Lead Have A Higher Heat Capacity Than Water.
From pressbooks.bccampus.ca
Water has a high heat capacity Chemistry for Biology 1190 Students Does Lead Have A Higher Heat Capacity Than Water Specific heat capacity is the measurement of how much energy (in j). Lead 0.128 water has a particularly high specific heat compared to many other substances. How do the specific heats of metals compare with water? It is the thermal capacity per unit mass which is. The heat capacity of an object depends both on its mass and its chemical. Does Lead Have A Higher Heat Capacity Than Water.
From ar.inspiredpencil.com
Common Heat Capacities Does Lead Have A Higher Heat Capacity Than Water Specific heat capacity is the measurement of how much energy (in j). Lead 0.128 water has a particularly high specific heat compared to many other substances. The heat capacity of an object depends both on its mass and its chemical composition. It is the thermal capacity per unit mass which is. Because of its much larger mass,. Its specific heat. Does Lead Have A Higher Heat Capacity Than Water.
From www.animalia-life.club
Specific Heat Chart Of Common Substances Does Lead Have A Higher Heat Capacity Than Water Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. How do the specific heats of metals compare with water? Lead is used because its specific thermal capacity is about 1/30 that of water. Specific heat of lead is 0.13 j/g k. It is the thermal capacity per unit mass which is. The reason is that. Does Lead Have A Higher Heat Capacity Than Water.
From studymind.co.uk
Heat Capacity Study Mind Does Lead Have A Higher Heat Capacity Than Water Because of its much larger mass,. The specific heat of metals are lower than that of water. It is the thermal capacity per unit mass which is. Lead 0.128 water has a particularly high specific heat compared to many other substances. Specific heat capacity is the measurement of how much energy (in j). How can the heat capacity of a. Does Lead Have A Higher Heat Capacity Than Water.
From classschoolwithstands.z21.web.core.windows.net
High Specific Heat In Water Does Lead Have A Higher Heat Capacity Than Water The reason is that water has a greater specific heat than most common substances and thus undergoes a small temperature change for a. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Lead 0.128 water has a particularly high specific heat compared to many other substances. Specific heat of lead is 0.13 j/g k. Specific. Does Lead Have A Higher Heat Capacity Than Water.
From www.slideserve.com
PPT Notes Water and its Special Properties PowerPoint Presentation Does Lead Have A Higher Heat Capacity Than Water Specific heat of lead is 0.13 j/g k. How do the specific heats of metals compare with water? Lead is used because its specific thermal capacity is about 1/30 that of water. Specific heat capacity is the measurement of how much energy (in j). When a given amount of heat is added to different. The specific heat of metals are. Does Lead Have A Higher Heat Capacity Than Water.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID6811859 Does Lead Have A Higher Heat Capacity Than Water Specific heat capacity is the measurement of how much energy (in j). Because of its much larger mass,. The reason is that water has a greater specific heat than most common substances and thus undergoes a small temperature change for a. Lead is used because its specific thermal capacity is about 1/30 that of water. How do the specific heats. Does Lead Have A Higher Heat Capacity Than Water.
From www.careerpower.in
What is Heat Capacity Definition, Equations, Examples and Types Does Lead Have A Higher Heat Capacity Than Water Lead 0.128 water has a particularly high specific heat compared to many other substances. How can the heat capacity of a lead sinker be determined? The heat capacity of an object depends both on its mass and its chemical composition. The specific heat of metals are lower than that of water. When a given amount of heat is added to. Does Lead Have A Higher Heat Capacity Than Water.
From www.slideserve.com
PPT High Specific Heat of Water PowerPoint Presentation, free Does Lead Have A Higher Heat Capacity Than Water The specific heat of metals are lower than that of water. The heat capacity of an object depends both on its mass and its chemical composition. Lead is used because its specific thermal capacity is about 1/30 that of water. Specific heat capacity is the measurement of how much energy (in j). When a given amount of heat is added. Does Lead Have A Higher Heat Capacity Than Water.
From ch302.cm.utexas.edu
heating curve Does Lead Have A Higher Heat Capacity Than Water Lead 0.128 water has a particularly high specific heat compared to many other substances. It is the thermal capacity per unit mass which is. Specific heat capacity is the measurement of how much energy (in j). Because of its much larger mass,. How do the specific heats of metals compare with water? Specific heat of lead is 0.13 j/g k.. Does Lead Have A Higher Heat Capacity Than Water.
From quizizz.com
Specific heat Capacity Science Quizizz Does Lead Have A Higher Heat Capacity Than Water Lead is used because its specific thermal capacity is about 1/30 that of water. How can the heat capacity of a lead sinker be determined? The reason is that water has a greater specific heat than most common substances and thus undergoes a small temperature change for a. It is the thermal capacity per unit mass which is. When a. Does Lead Have A Higher Heat Capacity Than Water.
From www.youtube.com
Reference Table Page 1Specific Heat of Common MaterialsHommocks Earth Does Lead Have A Higher Heat Capacity Than Water The heat capacity of an object depends both on its mass and its chemical composition. The reason is that water has a greater specific heat than most common substances and thus undergoes a small temperature change for a. The specific heat of metals are lower than that of water. It is the thermal capacity per unit mass which is. Its. Does Lead Have A Higher Heat Capacity Than Water.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer Does Lead Have A Higher Heat Capacity Than Water Lead is used because its specific thermal capacity is about 1/30 that of water. How can the heat capacity of a lead sinker be determined? The reason is that water has a greater specific heat than most common substances and thus undergoes a small temperature change for a. The heat capacity of an object depends both on its mass and. Does Lead Have A Higher Heat Capacity Than Water.
From www.tec-science.com
Specific heat capacity of selected substances tecscience Does Lead Have A Higher Heat Capacity Than Water Specific heat capacity is the measurement of how much energy (in j). When a given amount of heat is added to different. Lead 0.128 water has a particularly high specific heat compared to many other substances. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Specific heat of lead is 0.13 j/g k. The heat. Does Lead Have A Higher Heat Capacity Than Water.