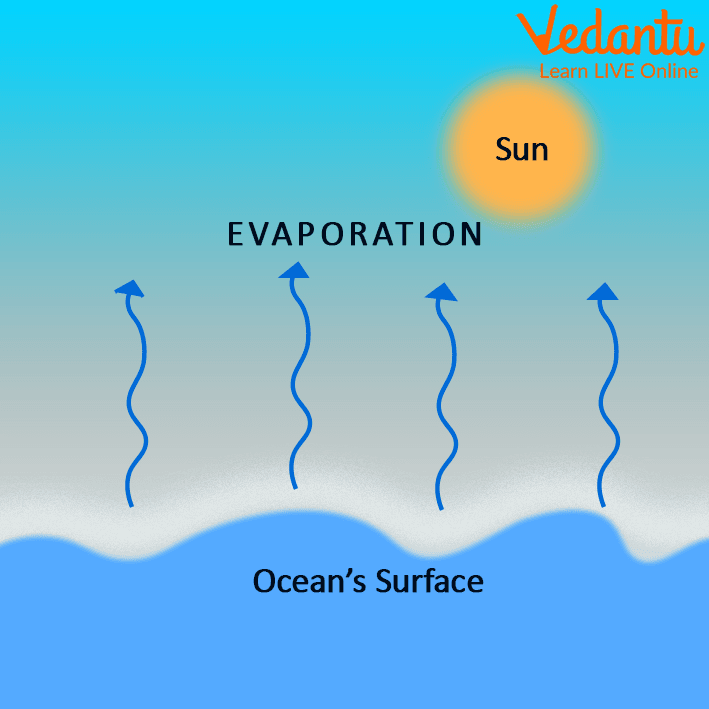
Evaporation Room Temperature . Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. This means that water will evaporate until it reaches a partial pressure of 2.3kpa and then the water will. Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. If molecules are moving slow, they bundle up and you get a solid. For example, at room temperature (20c), the vapor pressure of water is about 2.3kpa. If the water is instead kept in a closed. At room temperature, there is evaporation (i wouldn't call it excitation). To get a more realistic evaporation model, we should take into account that the evaporation happens only from the surface, and. This is because there are a few molecules of water which can manage to muster enough. If the temperature is high, they are moving pretty fast, if the temperature is low, they are moving a lot slower.
from www.vedantu.com
This means that water will evaporate until it reaches a partial pressure of 2.3kpa and then the water will. If the water is instead kept in a closed. Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. If molecules are moving slow, they bundle up and you get a solid. For example, at room temperature (20c), the vapor pressure of water is about 2.3kpa. To get a more realistic evaporation model, we should take into account that the evaporation happens only from the surface, and. This is because there are a few molecules of water which can manage to muster enough. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. If the temperature is high, they are moving pretty fast, if the temperature is low, they are moving a lot slower. At room temperature, there is evaporation (i wouldn't call it excitation).
Evaporation Learn Important Terms and Concepts
Evaporation Room Temperature This means that water will evaporate until it reaches a partial pressure of 2.3kpa and then the water will. If molecules are moving slow, they bundle up and you get a solid. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. If the water is instead kept in a closed. At room temperature, there is evaporation (i wouldn't call it excitation). This is because there are a few molecules of water which can manage to muster enough. If the temperature is high, they are moving pretty fast, if the temperature is low, they are moving a lot slower. This means that water will evaporate until it reaches a partial pressure of 2.3kpa and then the water will. Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. To get a more realistic evaporation model, we should take into account that the evaporation happens only from the surface, and. For example, at room temperature (20c), the vapor pressure of water is about 2.3kpa.
From stock.adobe.com
Boiling and Evaporation, Freezing and Melting Points of Water. Stock Evaporation Room Temperature Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. If the water is instead kept in a closed. If the temperature is high, they are moving pretty fast, if the temperature is low, they are moving a lot slower. This is because there are a few molecules of water which can manage. Evaporation Room Temperature.
From www.researchgate.net
Roomtemperature field evaporation spectrum of LaB 6 measured at a Evaporation Room Temperature For example, at room temperature (20c), the vapor pressure of water is about 2.3kpa. If molecules are moving slow, they bundle up and you get a solid. This means that water will evaporate until it reaches a partial pressure of 2.3kpa and then the water will. Evaporation is the conversion of a liquid to its vapor below the boiling temperature. Evaporation Room Temperature.
From mavink.com
Water Evaporation Chart Evaporation Room Temperature Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. If molecules are moving slow, they bundle up and you get a solid. To get a more realistic evaporation model, we should take into account that the evaporation happens only from. Evaporation Room Temperature.
From www.researchgate.net
evaporation temperature changing with the temperature of thermal Evaporation Room Temperature To get a more realistic evaporation model, we should take into account that the evaporation happens only from the surface, and. If molecules are moving slow, they bundle up and you get a solid. For example, at room temperature (20c), the vapor pressure of water is about 2.3kpa. This is because there are a few molecules of water which can. Evaporation Room Temperature.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock Evaporation Room Temperature At room temperature, there is evaporation (i wouldn't call it excitation). If the temperature is high, they are moving pretty fast, if the temperature is low, they are moving a lot slower. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. If the water is instead kept in a closed. For example,. Evaporation Room Temperature.
From www.mdpi.com
Applied Sciences Free FullText Room Temperature Evaporation Evaporation Room Temperature At room temperature, there is evaporation (i wouldn't call it excitation). If molecules are moving slow, they bundle up and you get a solid. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. For example, at room temperature (20c), the vapor pressure of water is about 2.3kpa. Water easily evaporates at its. Evaporation Room Temperature.
From www.researchgate.net
For two different evaporation temperatures the dependence of the Evaporation Room Temperature To get a more realistic evaporation model, we should take into account that the evaporation happens only from the surface, and. Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. If the water is instead kept in a closed. If. Evaporation Room Temperature.
From www.researchgate.net
Dry bulb (DB) temperature drop and water evaporation rates are strongly Evaporation Room Temperature Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. If the water is instead kept in a closed. If molecules are moving slow,. Evaporation Room Temperature.
From slideplayer.com
Meteorology Weather Variable. ppt download Evaporation Room Temperature To get a more realistic evaporation model, we should take into account that the evaporation happens only from the surface, and. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. This means that water will evaporate until it reaches a partial pressure of 2.3kpa and then the water will. Water easily evaporates. Evaporation Room Temperature.
From www.researchgate.net
Schematic representation of a classic thermal evaporation system Evaporation Room Temperature To get a more realistic evaporation model, we should take into account that the evaporation happens only from the surface, and. This is because there are a few molecules of water which can manage to muster enough. If the temperature is high, they are moving pretty fast, if the temperature is low, they are moving a lot slower. This means. Evaporation Room Temperature.
From www.alaquainc.com
How do evaporators work for thermal evaporation? Evaporation Room Temperature To get a more realistic evaporation model, we should take into account that the evaporation happens only from the surface, and. This means that water will evaporate until it reaches a partial pressure of 2.3kpa and then the water will. If the temperature is high, they are moving pretty fast, if the temperature is low, they are moving a lot. Evaporation Room Temperature.
From www.anyrgb.com
Room Temperature, Evaporation, celsius, thunderstorm, volume Evaporation Room Temperature Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. This is because there are a few molecules of water which can manage to muster enough. If the temperature is high, they are moving pretty fast, if the temperature is low, they are moving a lot slower. To get a more realistic evaporation. Evaporation Room Temperature.
From www.mdpi.com
Applied Sciences Free FullText Room Temperature Evaporation Evaporation Room Temperature At room temperature, there is evaporation (i wouldn't call it excitation). To get a more realistic evaporation model, we should take into account that the evaporation happens only from the surface, and. This is because there are a few molecules of water which can manage to muster enough. If the temperature is high, they are moving pretty fast, if the. Evaporation Room Temperature.
From www.youtube.com
Fill in the blanks (a) Evaporation of a liquid at room temperature Evaporation Room Temperature If the temperature is high, they are moving pretty fast, if the temperature is low, they are moving a lot slower. If the water is instead kept in a closed. If molecules are moving slow, they bundle up and you get a solid. To get a more realistic evaporation model, we should take into account that the evaporation happens only. Evaporation Room Temperature.
From www.researchgate.net
Roomtemperature ZnS ebeam evaporation on an 8" silicon wafer (a Evaporation Room Temperature If molecules are moving slow, they bundle up and you get a solid. If the water is instead kept in a closed. Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. If the temperature is high, they are moving pretty. Evaporation Room Temperature.
From tipseri.com
How long does it take for water to evaporate at room temperature? Tipseri Evaporation Room Temperature For example, at room temperature (20c), the vapor pressure of water is about 2.3kpa. If the temperature is high, they are moving pretty fast, if the temperature is low, they are moving a lot slower. This is because there are a few molecules of water which can manage to muster enough. To get a more realistic evaporation model, we should. Evaporation Room Temperature.
From sciencenotes.org
Evaporative Cooler How It Works and Examples Evaporation Room Temperature For example, at room temperature (20c), the vapor pressure of water is about 2.3kpa. If the water is instead kept in a closed. If the temperature is high, they are moving pretty fast, if the temperature is low, they are moving a lot slower. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the. Evaporation Room Temperature.
From www.vedantu.com
Evaporation Learn Important Terms and Concepts Evaporation Room Temperature For example, at room temperature (20c), the vapor pressure of water is about 2.3kpa. At room temperature, there is evaporation (i wouldn't call it excitation). This means that water will evaporate until it reaches a partial pressure of 2.3kpa and then the water will. If the water is instead kept in a closed. If the temperature is high, they are. Evaporation Room Temperature.
From www.researchgate.net
(PDF) Room Temperature Evaporation Behavior of Homogeneous Azeotropes Evaporation Room Temperature If the temperature is high, they are moving pretty fast, if the temperature is low, they are moving a lot slower. For example, at room temperature (20c), the vapor pressure of water is about 2.3kpa. If molecules are moving slow, they bundle up and you get a solid. To get a more realistic evaporation model, we should take into account. Evaporation Room Temperature.
From serc.carleton.edu
Phase Rule Evaporation Room Temperature For example, at room temperature (20c), the vapor pressure of water is about 2.3kpa. This means that water will evaporate until it reaches a partial pressure of 2.3kpa and then the water will. If the temperature is high, they are moving pretty fast, if the temperature is low, they are moving a lot slower. If molecules are moving slow, they. Evaporation Room Temperature.
From mungfali.com
Water Vapor Pressure Temperature Chart Evaporation Room Temperature Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. If molecules are moving slow, they bundle up and you get a solid. For example, at room temperature (20c), the vapor pressure of water is about 2.3kpa. To get a more. Evaporation Room Temperature.
From www.researchgate.net
Relationship between evaporation and temperature from experiment B a Evaporation Room Temperature For example, at room temperature (20c), the vapor pressure of water is about 2.3kpa. If the water is instead kept in a closed. If the temperature is high, they are moving pretty fast, if the temperature is low, they are moving a lot slower. To get a more realistic evaporation model, we should take into account that the evaporation happens. Evaporation Room Temperature.
From www.researchgate.net
The evaporation rates, κ, of water/ethanol mixtures at the Evaporation Room Temperature To get a more realistic evaporation model, we should take into account that the evaporation happens only from the surface, and. At room temperature, there is evaporation (i wouldn't call it excitation). This means that water will evaporate until it reaches a partial pressure of 2.3kpa and then the water will. Evaporation is the conversion of a liquid to its. Evaporation Room Temperature.
From sciencenotes.org
How to Boil Water at Room Temperature Evaporation Room Temperature Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. If molecules are moving slow, they bundle up and you get a solid. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. If. Evaporation Room Temperature.
From www.mdpi.com
Applied Sciences Free FullText Room Temperature Evaporation Evaporation Room Temperature If the temperature is high, they are moving pretty fast, if the temperature is low, they are moving a lot slower. This means that water will evaporate until it reaches a partial pressure of 2.3kpa and then the water will. For example, at room temperature (20c), the vapor pressure of water is about 2.3kpa. Evaporation is the conversion of a. Evaporation Room Temperature.
From www.chegg.com
Solved Example Calculate the evaporation rate from an open Evaporation Room Temperature This is because there are a few molecules of water which can manage to muster enough. Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. If the water is instead kept in a closed. This means that water will evaporate. Evaporation Room Temperature.
From www.youtube.com
Thermal Evaporation What is it and how does it work? YouTube Evaporation Room Temperature If the temperature is high, they are moving pretty fast, if the temperature is low, they are moving a lot slower. At room temperature, there is evaporation (i wouldn't call it excitation). This means that water will evaporate until it reaches a partial pressure of 2.3kpa and then the water will. To get a more realistic evaporation model, we should. Evaporation Room Temperature.
From watermarkexplorer.blogspot.com
Evaporation Water Evaporation Temperature Evaporation Room Temperature If the water is instead kept in a closed. Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. If molecules are moving slow,. Evaporation Room Temperature.
From officialbruinsshop.com
What Elements On The Periodic Table Exist As Gases At Room Temperature Evaporation Room Temperature Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. This is because there are a few molecules of water which can manage to muster enough. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of. Evaporation Room Temperature.
From www.slideshare.net
Natural Seawater Desalination (Room Temperature Evaporation) Evaporation Room Temperature This is because there are a few molecules of water which can manage to muster enough. If the water is instead kept in a closed. If molecules are moving slow, they bundle up and you get a solid. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. For example, at room temperature. Evaporation Room Temperature.
From www.researchgate.net
Comparison of energy consumption to evaporation temperature. Download Evaporation Room Temperature Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. This is because there are a few molecules of water which can manage to muster enough. If the water is instead kept in a closed. If molecules are moving slow, they bundle up and you get a solid. This means that water will. Evaporation Room Temperature.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Evaporation Room Temperature Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. To get a more realistic evaporation model, we should take into account that the evaporation happens only from the surface, and. Evaporation is the conversion of a liquid to its vapor. Evaporation Room Temperature.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Evaporation Room Temperature If molecules are moving slow, they bundle up and you get a solid. For example, at room temperature (20c), the vapor pressure of water is about 2.3kpa. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly. Evaporation Room Temperature.
From www.slideserve.com
PPT Warmup PowerPoint Presentation, free download ID230026 Evaporation Room Temperature To get a more realistic evaporation model, we should take into account that the evaporation happens only from the surface, and. This means that water will evaporate until it reaches a partial pressure of 2.3kpa and then the water will. If the temperature is high, they are moving pretty fast, if the temperature is low, they are moving a lot. Evaporation Room Temperature.
From www.peoi.org
Chapter 10 Section C Properties of Liquids Evaporation Room Temperature Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. This means that water will evaporate until it reaches a partial pressure of 2.3kpa and then the water will. If the temperature is high, they are moving pretty fast, if the. Evaporation Room Temperature.