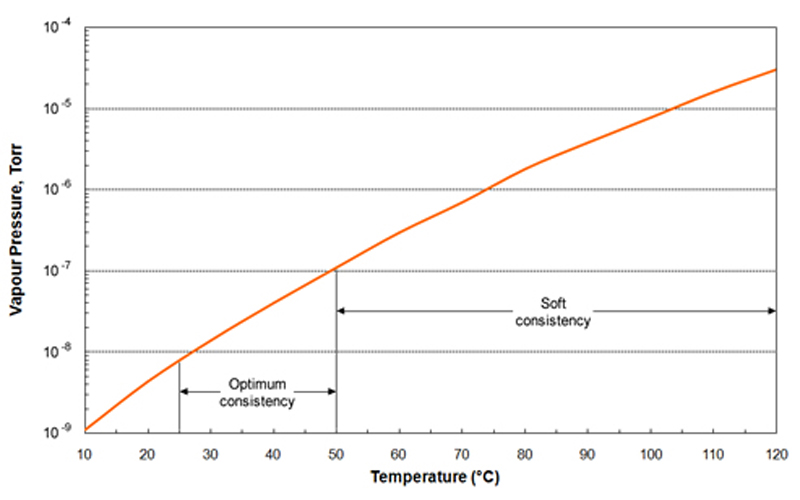
Vapor Pressure Vacuum . For a case where the condensing surface is not close to the evaporating surface, a pressure criterion for intensive evaporation is introduced, called the effective vacuum pressure,. The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, and the liquid changes into a vapor. Vapor pressure is defined by wikipedia as “the pressure exerted by a vapor on its condensed phases at a given temperature in a closed system”—a confusing. Vapor pressure is the pressure at which the vapor phase is in equilibrium with the solid or the liquid phase at a given temperature. • below this pressure, surface evaporation. Lide (ed), crc handbook of chemistry and physics, 84th edition, online version.
from ar.inspiredpencil.com
• below this pressure, surface evaporation. Vapor pressure is the pressure at which the vapor phase is in equilibrium with the solid or the liquid phase at a given temperature. Vapor pressure is defined by wikipedia as “the pressure exerted by a vapor on its condensed phases at a given temperature in a closed system”—a confusing. The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, and the liquid changes into a vapor. For a case where the condensing surface is not close to the evaporating surface, a pressure criterion for intensive evaporation is introduced, called the effective vacuum pressure,. Lide (ed), crc handbook of chemistry and physics, 84th edition, online version.
Equilibrium Vapor Pressure Vacuum
Vapor Pressure Vacuum • below this pressure, surface evaporation. Lide (ed), crc handbook of chemistry and physics, 84th edition, online version. The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, and the liquid changes into a vapor. Vapor pressure is the pressure at which the vapor phase is in equilibrium with the solid or the liquid phase at a given temperature. Vapor pressure is defined by wikipedia as “the pressure exerted by a vapor on its condensed phases at a given temperature in a closed system”—a confusing. • below this pressure, surface evaporation. For a case where the condensing surface is not close to the evaporating surface, a pressure criterion for intensive evaporation is introduced, called the effective vacuum pressure,.
From www.slideserve.com
PPT Vacuum Evaporation PowerPoint Presentation ID759145 Vapor Pressure Vacuum For a case where the condensing surface is not close to the evaporating surface, a pressure criterion for intensive evaporation is introduced, called the effective vacuum pressure,. Vapor pressure is the pressure at which the vapor phase is in equilibrium with the solid or the liquid phase at a given temperature. The boiling point of a substance is the temperature. Vapor Pressure Vacuum.
From www.slideserve.com
PPT Vacuum Technology PowerPoint Presentation, free download ID2999287 Vapor Pressure Vacuum Lide (ed), crc handbook of chemistry and physics, 84th edition, online version. The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, and the liquid changes into a vapor. Vapor pressure is the pressure at which the vapor phase is in equilibrium with the solid or the. Vapor Pressure Vacuum.
From ar.inspiredpencil.com
Equilibrium Vapor Pressure Vacuum Vapor Pressure Vacuum Lide (ed), crc handbook of chemistry and physics, 84th edition, online version. For a case where the condensing surface is not close to the evaporating surface, a pressure criterion for intensive evaporation is introduced, called the effective vacuum pressure,. Vapor pressure is the pressure at which the vapor phase is in equilibrium with the solid or the liquid phase at. Vapor Pressure Vacuum.
From ar.inspiredpencil.com
Equilibrium Vapor Pressure Vacuum Vapor Pressure Vacuum For a case where the condensing surface is not close to the evaporating surface, a pressure criterion for intensive evaporation is introduced, called the effective vacuum pressure,. Vapor pressure is defined by wikipedia as “the pressure exerted by a vapor on its condensed phases at a given temperature in a closed system”—a confusing. Lide (ed), crc handbook of chemistry and. Vapor Pressure Vacuum.
From www.researchgate.net
Methanol vapor pressure curve. Markers located at atmospheric pressure Vapor Pressure Vacuum • below this pressure, surface evaporation. Vapor pressure is defined by wikipedia as “the pressure exerted by a vapor on its condensed phases at a given temperature in a closed system”—a confusing. The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, and the liquid changes into. Vapor Pressure Vacuum.
From ar.inspiredpencil.com
Equilibrium Vapor Pressure Vacuum Vapor Pressure Vacuum The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, and the liquid changes into a vapor. Vapor pressure is defined by wikipedia as “the pressure exerted by a vapor on its condensed phases at a given temperature in a closed system”—a confusing. Lide (ed), crc handbook. Vapor Pressure Vacuum.
From ar.inspiredpencil.com
Equilibrium Vapor Pressure Vacuum Vapor Pressure Vacuum Vapor pressure is the pressure at which the vapor phase is in equilibrium with the solid or the liquid phase at a given temperature. Lide (ed), crc handbook of chemistry and physics, 84th edition, online version. • below this pressure, surface evaporation. The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals. Vapor Pressure Vacuum.
From ar.inspiredpencil.com
Equilibrium Vapor Pressure Vacuum Vapor Pressure Vacuum • below this pressure, surface evaporation. Vapor pressure is defined by wikipedia as “the pressure exerted by a vapor on its condensed phases at a given temperature in a closed system”—a confusing. For a case where the condensing surface is not close to the evaporating surface, a pressure criterion for intensive evaporation is introduced, called the effective vacuum pressure,. Lide. Vapor Pressure Vacuum.
From www.youtube.com
Vapor Pressure and Boiling YouTube Vapor Pressure Vacuum The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, and the liquid changes into a vapor. • below this pressure, surface evaporation. Vapor pressure is defined by wikipedia as “the pressure exerted by a vapor on its condensed phases at a given temperature in a closed. Vapor Pressure Vacuum.
From www.slideserve.com
PPT Vacuum Technology PowerPoint Presentation, free download ID2999287 Vapor Pressure Vacuum Lide (ed), crc handbook of chemistry and physics, 84th edition, online version. For a case where the condensing surface is not close to the evaporating surface, a pressure criterion for intensive evaporation is introduced, called the effective vacuum pressure,. Vapor pressure is the pressure at which the vapor phase is in equilibrium with the solid or the liquid phase at. Vapor Pressure Vacuum.
From www.chegg.com
Solved Shown below is the vapor pressure curve for water Vapor Pressure Vacuum • below this pressure, surface evaporation. Lide (ed), crc handbook of chemistry and physics, 84th edition, online version. Vapor pressure is defined by wikipedia as “the pressure exerted by a vapor on its condensed phases at a given temperature in a closed system”—a confusing. Vapor pressure is the pressure at which the vapor phase is in equilibrium with the solid. Vapor Pressure Vacuum.
From ar.inspiredpencil.com
Equilibrium Vapor Pressure Vacuum Vapor Pressure Vacuum • below this pressure, surface evaporation. Lide (ed), crc handbook of chemistry and physics, 84th edition, online version. For a case where the condensing surface is not close to the evaporating surface, a pressure criterion for intensive evaporation is introduced, called the effective vacuum pressure,. Vapor pressure is defined by wikipedia as “the pressure exerted by a vapor on its. Vapor Pressure Vacuum.
From www.slideserve.com
PPT Vacuum Technology PowerPoint Presentation, free download ID2999287 Vapor Pressure Vacuum Vapor pressure is defined by wikipedia as “the pressure exerted by a vapor on its condensed phases at a given temperature in a closed system”—a confusing. • below this pressure, surface evaporation. For a case where the condensing surface is not close to the evaporating surface, a pressure criterion for intensive evaporation is introduced, called the effective vacuum pressure,. Lide. Vapor Pressure Vacuum.
From www.youtube.com
What is Atmospheric pressure, Gauge pressure, Absolute Pressure, Vacuum Vapor Pressure Vacuum Vapor pressure is defined by wikipedia as “the pressure exerted by a vapor on its condensed phases at a given temperature in a closed system”—a confusing. Vapor pressure is the pressure at which the vapor phase is in equilibrium with the solid or the liquid phase at a given temperature. • below this pressure, surface evaporation. For a case where. Vapor Pressure Vacuum.
From www.researchgate.net
The vapor pressure of ethanol vs. the normal boilingpoint temperature Vapor Pressure Vacuum The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, and the liquid changes into a vapor. For a case where the condensing surface is not close to the evaporating surface, a pressure criterion for intensive evaporation is introduced, called the effective vacuum pressure,. Lide (ed), crc. Vapor Pressure Vacuum.
From engineerexcel.com
Vapor Pressure of Water Explained EngineerExcel Vapor Pressure Vacuum Vapor pressure is the pressure at which the vapor phase is in equilibrium with the solid or the liquid phase at a given temperature. Vapor pressure is defined by wikipedia as “the pressure exerted by a vapor on its condensed phases at a given temperature in a closed system”—a confusing. The boiling point of a substance is the temperature at. Vapor Pressure Vacuum.
From www.slideserve.com
PPT VAPOR PRESSURE OF WATER PowerPoint Presentation, free download Vapor Pressure Vacuum Lide (ed), crc handbook of chemistry and physics, 84th edition, online version. Vapor pressure is the pressure at which the vapor phase is in equilibrium with the solid or the liquid phase at a given temperature. • below this pressure, surface evaporation. Vapor pressure is defined by wikipedia as “the pressure exerted by a vapor on its condensed phases at. Vapor Pressure Vacuum.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID1025977 Vapor Pressure Vacuum For a case where the condensing surface is not close to the evaporating surface, a pressure criterion for intensive evaporation is introduced, called the effective vacuum pressure,. Vapor pressure is the pressure at which the vapor phase is in equilibrium with the solid or the liquid phase at a given temperature. • below this pressure, surface evaporation. The boiling point. Vapor Pressure Vacuum.
From cacheby.com
Vapor Pressure Vacuum Controller 증기 압력 진공 컨트롤러 캐시바이 Vapor Pressure Vacuum Vapor pressure is the pressure at which the vapor phase is in equilibrium with the solid or the liquid phase at a given temperature. • below this pressure, surface evaporation. The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, and the liquid changes into a vapor.. Vapor Pressure Vacuum.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube Vapor Pressure Vacuum Lide (ed), crc handbook of chemistry and physics, 84th edition, online version. Vapor pressure is the pressure at which the vapor phase is in equilibrium with the solid or the liquid phase at a given temperature. For a case where the condensing surface is not close to the evaporating surface, a pressure criterion for intensive evaporation is introduced, called the. Vapor Pressure Vacuum.
From chem.libretexts.org
Chapter 11.4 Vapor Pressure Chemistry LibreTexts Vapor Pressure Vacuum Vapor pressure is the pressure at which the vapor phase is in equilibrium with the solid or the liquid phase at a given temperature. Vapor pressure is defined by wikipedia as “the pressure exerted by a vapor on its condensed phases at a given temperature in a closed system”—a confusing. Lide (ed), crc handbook of chemistry and physics, 84th edition,. Vapor Pressure Vacuum.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 Vapor Pressure Vacuum Vapor pressure is defined by wikipedia as “the pressure exerted by a vapor on its condensed phases at a given temperature in a closed system”—a confusing. For a case where the condensing surface is not close to the evaporating surface, a pressure criterion for intensive evaporation is introduced, called the effective vacuum pressure,. The boiling point of a substance is. Vapor Pressure Vacuum.
From www.researchgate.net
Equilibrium vapour pressures for In and Zn in the 200 800 ° C Vapor Pressure Vacuum Vapor pressure is defined by wikipedia as “the pressure exerted by a vapor on its condensed phases at a given temperature in a closed system”—a confusing. • below this pressure, surface evaporation. Lide (ed), crc handbook of chemistry and physics, 84th edition, online version. Vapor pressure is the pressure at which the vapor phase is in equilibrium with the solid. Vapor Pressure Vacuum.
From vacaero.com
Simple Physics for the High Vacuum Processing Industry Vapor Pressure Vacuum Vapor pressure is the pressure at which the vapor phase is in equilibrium with the solid or the liquid phase at a given temperature. The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, and the liquid changes into a vapor. For a case where the condensing. Vapor Pressure Vacuum.
From mavink.com
Water Vapour Pressure Chart Vapor Pressure Vacuum Vapor pressure is the pressure at which the vapor phase is in equilibrium with the solid or the liquid phase at a given temperature. Vapor pressure is defined by wikipedia as “the pressure exerted by a vapor on its condensed phases at a given temperature in a closed system”—a confusing. • below this pressure, surface evaporation. Lide (ed), crc handbook. Vapor Pressure Vacuum.
From dhilreefer.blogspot.com
Vapor Pressure Curves For Vacuum Brazing Vapor Pressure Vacuum The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, and the liquid changes into a vapor. • below this pressure, surface evaporation. For a case where the condensing surface is not close to the evaporating surface, a pressure criterion for intensive evaporation is introduced, called the. Vapor Pressure Vacuum.
From ar.inspiredpencil.com
Equilibrium Vapor Pressure Vacuum Vapor Pressure Vacuum Lide (ed), crc handbook of chemistry and physics, 84th edition, online version. For a case where the condensing surface is not close to the evaporating surface, a pressure criterion for intensive evaporation is introduced, called the effective vacuum pressure,. The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding. Vapor Pressure Vacuum.
From ar.inspiredpencil.com
Equilibrium Vapor Pressure Vacuum Vapor Pressure Vacuum Lide (ed), crc handbook of chemistry and physics, 84th edition, online version. • below this pressure, surface evaporation. Vapor pressure is the pressure at which the vapor phase is in equilibrium with the solid or the liquid phase at a given temperature. Vapor pressure is defined by wikipedia as “the pressure exerted by a vapor on its condensed phases at. Vapor Pressure Vacuum.
From ar.inspiredpencil.com
Equilibrium Vapor Pressure Vacuum Vapor Pressure Vacuum • below this pressure, surface evaporation. The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, and the liquid changes into a vapor. Vapor pressure is defined by wikipedia as “the pressure exerted by a vapor on its condensed phases at a given temperature in a closed. Vapor Pressure Vacuum.
From sciencenotes.org
Clausius Clapeyron Equation Vapor Pressure Vacuum • below this pressure, surface evaporation. Vapor pressure is the pressure at which the vapor phase is in equilibrium with the solid or the liquid phase at a given temperature. The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, and the liquid changes into a vapor.. Vapor Pressure Vacuum.
From ar.inspiredpencil.com
Equilibrium Vapor Pressure Vacuum Vapor Pressure Vacuum The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, and the liquid changes into a vapor. Vapor pressure is defined by wikipedia as “the pressure exerted by a vapor on its condensed phases at a given temperature in a closed system”—a confusing. Vapor pressure is the. Vapor Pressure Vacuum.
From ar.inspiredpencil.com
Equilibrium Vapor Pressure Vacuum Vapor Pressure Vacuum For a case where the condensing surface is not close to the evaporating surface, a pressure criterion for intensive evaporation is introduced, called the effective vacuum pressure,. Vapor pressure is defined by wikipedia as “the pressure exerted by a vapor on its condensed phases at a given temperature in a closed system”—a confusing. Vapor pressure is the pressure at which. Vapor Pressure Vacuum.
From ar.inspiredpencil.com
Equilibrium Vapor Pressure Vacuum Vapor Pressure Vacuum For a case where the condensing surface is not close to the evaporating surface, a pressure criterion for intensive evaporation is introduced, called the effective vacuum pressure,. Vapor pressure is the pressure at which the vapor phase is in equilibrium with the solid or the liquid phase at a given temperature. Lide (ed), crc handbook of chemistry and physics, 84th. Vapor Pressure Vacuum.
From www.doubtnut.com
The vapour pressure curves of the same solute in the same solvent are Vapor Pressure Vacuum Lide (ed), crc handbook of chemistry and physics, 84th edition, online version. For a case where the condensing surface is not close to the evaporating surface, a pressure criterion for intensive evaporation is introduced, called the effective vacuum pressure,. Vapor pressure is the pressure at which the vapor phase is in equilibrium with the solid or the liquid phase at. Vapor Pressure Vacuum.
From ar.inspiredpencil.com
Equilibrium Vapor Pressure Vacuum Vapor Pressure Vacuum Vapor pressure is defined by wikipedia as “the pressure exerted by a vapor on its condensed phases at a given temperature in a closed system”—a confusing. Vapor pressure is the pressure at which the vapor phase is in equilibrium with the solid or the liquid phase at a given temperature. The boiling point of a substance is the temperature at. Vapor Pressure Vacuum.