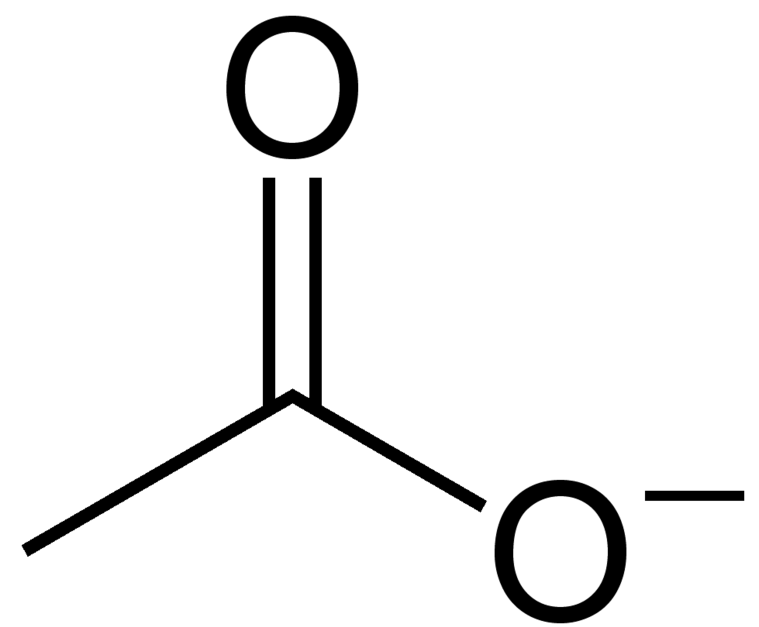
Acetic Acid Vs Acetate . Acetate and acetic acid are two chemical compounds that are closely related and often confused due to their similar names. Acetate is a monocarboxylic acid anion resulting from the removal of a proton from the carboxy group of acetic acid. It has a role as a human metabolite and a saccharomyces cerevisiae. Acetic acid (ch3cooh), the most important of the carboxylic acids. It is a buffer because it contains both. Acetic acid is an organic compound with the formula ch3cooh, known for its sour taste and pungent smell, commonly found in vinegar. A dilute (approximately 5 percent by volume) solution of acetic acid produced by fermentation and. A mixture of acetic acid and sodium acetate is acidic because the k a of acetic acid is greater than the k b of its conjugate base acetate. In summary, while acetic acid and acetate are chemically related, they differ in their forms, properties, and applications.
from
A dilute (approximately 5 percent by volume) solution of acetic acid produced by fermentation and. A mixture of acetic acid and sodium acetate is acidic because the k a of acetic acid is greater than the k b of its conjugate base acetate. Acetate is a monocarboxylic acid anion resulting from the removal of a proton from the carboxy group of acetic acid. Acetate and acetic acid are two chemical compounds that are closely related and often confused due to their similar names. It is a buffer because it contains both. Acetic acid is an organic compound with the formula ch3cooh, known for its sour taste and pungent smell, commonly found in vinegar. Acetic acid (ch3cooh), the most important of the carboxylic acids. It has a role as a human metabolite and a saccharomyces cerevisiae. In summary, while acetic acid and acetate are chemically related, they differ in their forms, properties, and applications.
Acetic Acid Vs Acetate Acetate is a monocarboxylic acid anion resulting from the removal of a proton from the carboxy group of acetic acid. In summary, while acetic acid and acetate are chemically related, they differ in their forms, properties, and applications. It has a role as a human metabolite and a saccharomyces cerevisiae. A dilute (approximately 5 percent by volume) solution of acetic acid produced by fermentation and. A mixture of acetic acid and sodium acetate is acidic because the k a of acetic acid is greater than the k b of its conjugate base acetate. Acetic acid is an organic compound with the formula ch3cooh, known for its sour taste and pungent smell, commonly found in vinegar. Acetate is a monocarboxylic acid anion resulting from the removal of a proton from the carboxy group of acetic acid. It is a buffer because it contains both. Acetic acid (ch3cooh), the most important of the carboxylic acids. Acetate and acetic acid are two chemical compounds that are closely related and often confused due to their similar names.
From
Acetic Acid Vs Acetate Acetate and acetic acid are two chemical compounds that are closely related and often confused due to their similar names. A dilute (approximately 5 percent by volume) solution of acetic acid produced by fermentation and. It is a buffer because it contains both. It has a role as a human metabolite and a saccharomyces cerevisiae. Acetic acid is an organic. Acetic Acid Vs Acetate.
From www.slideserve.com
PPT Equilibrium Acids and Bases PowerPoint Presentation, free download ID4499515 Acetic Acid Vs Acetate A mixture of acetic acid and sodium acetate is acidic because the k a of acetic acid is greater than the k b of its conjugate base acetate. It has a role as a human metabolite and a saccharomyces cerevisiae. Acetic acid is an organic compound with the formula ch3cooh, known for its sour taste and pungent smell, commonly found. Acetic Acid Vs Acetate.
From
Acetic Acid Vs Acetate Acetic acid (ch3cooh), the most important of the carboxylic acids. A dilute (approximately 5 percent by volume) solution of acetic acid produced by fermentation and. In summary, while acetic acid and acetate are chemically related, they differ in their forms, properties, and applications. A mixture of acetic acid and sodium acetate is acidic because the k a of acetic acid. Acetic Acid Vs Acetate.
From
Acetic Acid Vs Acetate A dilute (approximately 5 percent by volume) solution of acetic acid produced by fermentation and. Acetic acid is an organic compound with the formula ch3cooh, known for its sour taste and pungent smell, commonly found in vinegar. Acetic acid (ch3cooh), the most important of the carboxylic acids. It is a buffer because it contains both. Acetate is a monocarboxylic acid. Acetic Acid Vs Acetate.
From
Acetic Acid Vs Acetate A mixture of acetic acid and sodium acetate is acidic because the k a of acetic acid is greater than the k b of its conjugate base acetate. Acetic acid (ch3cooh), the most important of the carboxylic acids. In summary, while acetic acid and acetate are chemically related, they differ in their forms, properties, and applications. It has a role. Acetic Acid Vs Acetate.
From www.alamy.com
Ethyl acetate, ethyl ethanoate, C4H8O2 molecule. It is acetate ester formed between acetic acid Acetic Acid Vs Acetate Acetic acid (ch3cooh), the most important of the carboxylic acids. It is a buffer because it contains both. Acetate is a monocarboxylic acid anion resulting from the removal of a proton from the carboxy group of acetic acid. Acetic acid is an organic compound with the formula ch3cooh, known for its sour taste and pungent smell, commonly found in vinegar.. Acetic Acid Vs Acetate.
From pubs.sciepub.com
Figure 2. Sources and fate of active acetate (acetyl CoA). Acetyl CoA is produced during Acetic Acid Vs Acetate A dilute (approximately 5 percent by volume) solution of acetic acid produced by fermentation and. It is a buffer because it contains both. Acetate and acetic acid are two chemical compounds that are closely related and often confused due to their similar names. Acetic acid is an organic compound with the formula ch3cooh, known for its sour taste and pungent. Acetic Acid Vs Acetate.
From
Acetic Acid Vs Acetate It is a buffer because it contains both. Acetic acid is an organic compound with the formula ch3cooh, known for its sour taste and pungent smell, commonly found in vinegar. Acetic acid (ch3cooh), the most important of the carboxylic acids. Acetate and acetic acid are two chemical compounds that are closely related and often confused due to their similar names.. Acetic Acid Vs Acetate.
From fr.wikidoc.org
Acetic acid wikidoc Acetic Acid Vs Acetate A dilute (approximately 5 percent by volume) solution of acetic acid produced by fermentation and. Acetate is a monocarboxylic acid anion resulting from the removal of a proton from the carboxy group of acetic acid. Acetic acid is an organic compound with the formula ch3cooh, known for its sour taste and pungent smell, commonly found in vinegar. In summary, while. Acetic Acid Vs Acetate.
From
Acetic Acid Vs Acetate Acetate is a monocarboxylic acid anion resulting from the removal of a proton from the carboxy group of acetic acid. It is a buffer because it contains both. Acetic acid (ch3cooh), the most important of the carboxylic acids. It has a role as a human metabolite and a saccharomyces cerevisiae. Acetic acid is an organic compound with the formula ch3cooh,. Acetic Acid Vs Acetate.
From askanydifference.com
Acetone vs Acetic Acid Difference and Comparison Acetic Acid Vs Acetate Acetate is a monocarboxylic acid anion resulting from the removal of a proton from the carboxy group of acetic acid. Acetic acid is an organic compound with the formula ch3cooh, known for its sour taste and pungent smell, commonly found in vinegar. In summary, while acetic acid and acetate are chemically related, they differ in their forms, properties, and applications.. Acetic Acid Vs Acetate.
From
Acetic Acid Vs Acetate Acetate and acetic acid are two chemical compounds that are closely related and often confused due to their similar names. Acetate is a monocarboxylic acid anion resulting from the removal of a proton from the carboxy group of acetic acid. A mixture of acetic acid and sodium acetate is acidic because the k a of acetic acid is greater than. Acetic Acid Vs Acetate.
From askanydifference.com
Acetone vs Acetic Acid Difference and Comparison Acetic Acid Vs Acetate Acetic acid (ch3cooh), the most important of the carboxylic acids. It is a buffer because it contains both. A dilute (approximately 5 percent by volume) solution of acetic acid produced by fermentation and. A mixture of acetic acid and sodium acetate is acidic because the k a of acetic acid is greater than the k b of its conjugate base. Acetic Acid Vs Acetate.
From
Acetic Acid Vs Acetate A dilute (approximately 5 percent by volume) solution of acetic acid produced by fermentation and. It is a buffer because it contains both. A mixture of acetic acid and sodium acetate is acidic because the k a of acetic acid is greater than the k b of its conjugate base acetate. Acetate and acetic acid are two chemical compounds that. Acetic Acid Vs Acetate.
From pediaa.com
Difference Between Acetic Acid and Citric Acid Definition, Properties, Applications Acetic Acid Vs Acetate In summary, while acetic acid and acetate are chemically related, they differ in their forms, properties, and applications. Acetate and acetic acid are two chemical compounds that are closely related and often confused due to their similar names. Acetic acid (ch3cooh), the most important of the carboxylic acids. A mixture of acetic acid and sodium acetate is acidic because the. Acetic Acid Vs Acetate.
From
Acetic Acid Vs Acetate Acetate and acetic acid are two chemical compounds that are closely related and often confused due to their similar names. A mixture of acetic acid and sodium acetate is acidic because the k a of acetic acid is greater than the k b of its conjugate base acetate. In summary, while acetic acid and acetate are chemically related, they differ. Acetic Acid Vs Acetate.
From
Acetic Acid Vs Acetate Acetate and acetic acid are two chemical compounds that are closely related and often confused due to their similar names. Acetic acid (ch3cooh), the most important of the carboxylic acids. It is a buffer because it contains both. It has a role as a human metabolite and a saccharomyces cerevisiae. In summary, while acetic acid and acetate are chemically related,. Acetic Acid Vs Acetate.
From differencebtw.com
Acetic Acid vs. Acetate Know the Difference Acetic Acid Vs Acetate In summary, while acetic acid and acetate are chemically related, they differ in their forms, properties, and applications. A dilute (approximately 5 percent by volume) solution of acetic acid produced by fermentation and. A mixture of acetic acid and sodium acetate is acidic because the k a of acetic acid is greater than the k b of its conjugate base. Acetic Acid Vs Acetate.
From www.slchemtech.com
Difference Between Acetate vs Acetone Acetic Acid Vs Acetate Acetate is a monocarboxylic acid anion resulting from the removal of a proton from the carboxy group of acetic acid. Acetic acid (ch3cooh), the most important of the carboxylic acids. Acetic acid is an organic compound with the formula ch3cooh, known for its sour taste and pungent smell, commonly found in vinegar. It is a buffer because it contains both.. Acetic Acid Vs Acetate.
From
Acetic Acid Vs Acetate A dilute (approximately 5 percent by volume) solution of acetic acid produced by fermentation and. Acetic acid (ch3cooh), the most important of the carboxylic acids. Acetate is a monocarboxylic acid anion resulting from the removal of a proton from the carboxy group of acetic acid. Acetate and acetic acid are two chemical compounds that are closely related and often confused. Acetic Acid Vs Acetate.
From
Acetic Acid Vs Acetate Acetic acid (ch3cooh), the most important of the carboxylic acids. Acetate is a monocarboxylic acid anion resulting from the removal of a proton from the carboxy group of acetic acid. A mixture of acetic acid and sodium acetate is acidic because the k a of acetic acid is greater than the k b of its conjugate base acetate. It is. Acetic Acid Vs Acetate.
From
Acetic Acid Vs Acetate Acetic acid (ch3cooh), the most important of the carboxylic acids. Acetate and acetic acid are two chemical compounds that are closely related and often confused due to their similar names. Acetic acid is an organic compound with the formula ch3cooh, known for its sour taste and pungent smell, commonly found in vinegar. It has a role as a human metabolite. Acetic Acid Vs Acetate.
From mungfali.com
Chemical Structure Of Acetic Acid Acetic Acid Vs Acetate Acetate is a monocarboxylic acid anion resulting from the removal of a proton from the carboxy group of acetic acid. Acetic acid is an organic compound with the formula ch3cooh, known for its sour taste and pungent smell, commonly found in vinegar. A dilute (approximately 5 percent by volume) solution of acetic acid produced by fermentation and. It is a. Acetic Acid Vs Acetate.
From www.slideserve.com
PPT Formal Charge PowerPoint Presentation, free download ID338614 Acetic Acid Vs Acetate It is a buffer because it contains both. Acetic acid (ch3cooh), the most important of the carboxylic acids. Acetic acid is an organic compound with the formula ch3cooh, known for its sour taste and pungent smell, commonly found in vinegar. Acetate and acetic acid are two chemical compounds that are closely related and often confused due to their similar names.. Acetic Acid Vs Acetate.
From www.youtube.com
Difference between Acetone and Acetic Acid YouTube Acetic Acid Vs Acetate Acetic acid is an organic compound with the formula ch3cooh, known for its sour taste and pungent smell, commonly found in vinegar. Acetate and acetic acid are two chemical compounds that are closely related and often confused due to their similar names. Acetate is a monocarboxylic acid anion resulting from the removal of a proton from the carboxy group of. Acetic Acid Vs Acetate.
From
Acetic Acid Vs Acetate Acetic acid (ch3cooh), the most important of the carboxylic acids. Acetate and acetic acid are two chemical compounds that are closely related and often confused due to their similar names. A dilute (approximately 5 percent by volume) solution of acetic acid produced by fermentation and. Acetate is a monocarboxylic acid anion resulting from the removal of a proton from the. Acetic Acid Vs Acetate.
From askanydifference.com
Acetone vs Acetic Acid Difference and Comparison Acetic Acid Vs Acetate It is a buffer because it contains both. A mixture of acetic acid and sodium acetate is acidic because the k a of acetic acid is greater than the k b of its conjugate base acetate. Acetate and acetic acid are two chemical compounds that are closely related and often confused due to their similar names. Acetate is a monocarboxylic. Acetic Acid Vs Acetate.
From www.sciencephoto.com
Acetic acid molecule Stock Image F012/5730 Science Photo Library Acetic Acid Vs Acetate Acetic acid (ch3cooh), the most important of the carboxylic acids. A dilute (approximately 5 percent by volume) solution of acetic acid produced by fermentation and. It has a role as a human metabolite and a saccharomyces cerevisiae. Acetate is a monocarboxylic acid anion resulting from the removal of a proton from the carboxy group of acetic acid. Acetate and acetic. Acetic Acid Vs Acetate.
From pediaa.com
Difference Between Acetic Acid and Glacial Acetic Acid Properties, Structure, Uses Acetic Acid Vs Acetate A mixture of acetic acid and sodium acetate is acidic because the k a of acetic acid is greater than the k b of its conjugate base acetate. Acetate and acetic acid are two chemical compounds that are closely related and often confused due to their similar names. Acetate is a monocarboxylic acid anion resulting from the removal of a. Acetic Acid Vs Acetate.
From www.differencebetween.com
Difference Between Acetic Acid and Acetate Compare the Difference Between Similar Terms Acetic Acid Vs Acetate Acetate is a monocarboxylic acid anion resulting from the removal of a proton from the carboxy group of acetic acid. In summary, while acetic acid and acetate are chemically related, they differ in their forms, properties, and applications. It has a role as a human metabolite and a saccharomyces cerevisiae. A mixture of acetic acid and sodium acetate is acidic. Acetic Acid Vs Acetate.
From www.researchgate.net
Reaction pathway of acetic acid/acetate and ZnO cluster forming metal... Download Scientific Acetic Acid Vs Acetate A mixture of acetic acid and sodium acetate is acidic because the k a of acetic acid is greater than the k b of its conjugate base acetate. It is a buffer because it contains both. Acetic acid (ch3cooh), the most important of the carboxylic acids. A dilute (approximately 5 percent by volume) solution of acetic acid produced by fermentation. Acetic Acid Vs Acetate.
From
Acetic Acid Vs Acetate It is a buffer because it contains both. Acetic acid (ch3cooh), the most important of the carboxylic acids. A dilute (approximately 5 percent by volume) solution of acetic acid produced by fermentation and. It has a role as a human metabolite and a saccharomyces cerevisiae. Acetate and acetic acid are two chemical compounds that are closely related and often confused. Acetic Acid Vs Acetate.
From
Acetic Acid Vs Acetate Acetic acid is an organic compound with the formula ch3cooh, known for its sour taste and pungent smell, commonly found in vinegar. Acetate and acetic acid are two chemical compounds that are closely related and often confused due to their similar names. It is a buffer because it contains both. A dilute (approximately 5 percent by volume) solution of acetic. Acetic Acid Vs Acetate.
From saylordotorg.github.io
Aqueous AcidBase Equilibriums Acetic Acid Vs Acetate Acetate is a monocarboxylic acid anion resulting from the removal of a proton from the carboxy group of acetic acid. In summary, while acetic acid and acetate are chemically related, they differ in their forms, properties, and applications. Acetate and acetic acid are two chemical compounds that are closely related and often confused due to their similar names. Acetic acid. Acetic Acid Vs Acetate.
From pediaa.com
Difference Between Acetic Acid and Glacial Acetic Acid Properties, Structure, Uses Acetic Acid Vs Acetate Acetic acid (ch3cooh), the most important of the carboxylic acids. A mixture of acetic acid and sodium acetate is acidic because the k a of acetic acid is greater than the k b of its conjugate base acetate. Acetic acid is an organic compound with the formula ch3cooh, known for its sour taste and pungent smell, commonly found in vinegar.. Acetic Acid Vs Acetate.