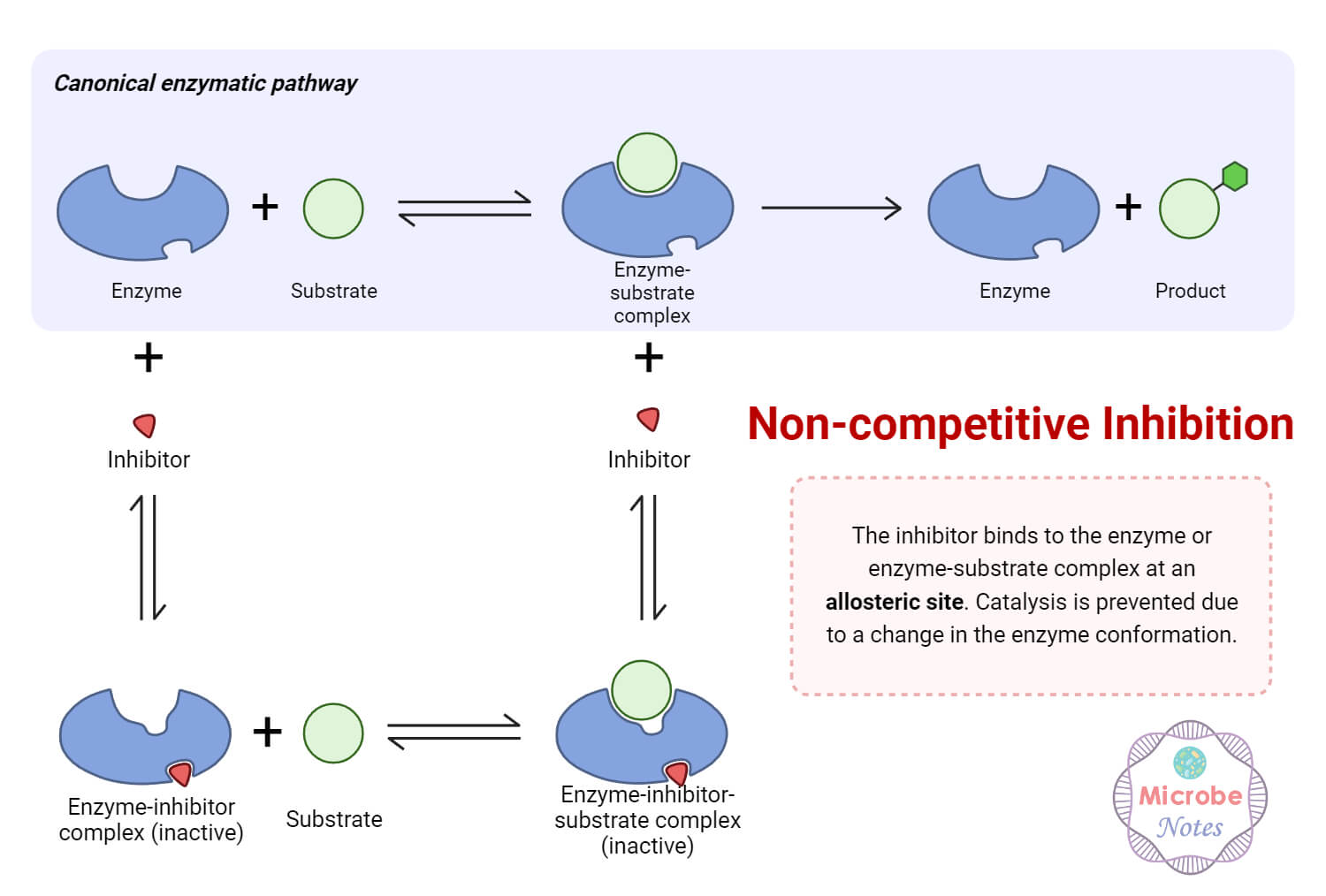
In Mixed Inhibition The Allosteric Effect Effects . An allosteric inhibitor decreases activity by binding to an. Many regulatory enzymes also display allosteric activation, and a single effector often acts in opposite directions on opposing reactions: The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. Allosteric interactions occur when the binding of a ligand (not necessarily a substrate) to a. In mixed inhibition, the inhibitor binds to an allosteric site, i.e. For example, in many organisms. If s and i bound to different sites, and s bound to e and produced a conformational change in e such that i could not bind (and vice. A site different from the active site where the substrate binds.
from microbenotes.com
A site different from the active site where the substrate binds. Allosteric interactions occur when the binding of a ligand (not necessarily a substrate) to a. In mixed inhibition, the inhibitor binds to an allosteric site, i.e. For example, in many organisms. If s and i bound to different sites, and s bound to e and produced a conformational change in e such that i could not bind (and vice. Many regulatory enzymes also display allosteric activation, and a single effector often acts in opposite directions on opposing reactions: The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. An allosteric inhibitor decreases activity by binding to an.
Allosteric Inhibition Mechanism, Cooperativity, Examples
In Mixed Inhibition The Allosteric Effect Effects An allosteric inhibitor decreases activity by binding to an. If s and i bound to different sites, and s bound to e and produced a conformational change in e such that i could not bind (and vice. Allosteric interactions occur when the binding of a ligand (not necessarily a substrate) to a. For example, in many organisms. An allosteric inhibitor decreases activity by binding to an. In mixed inhibition, the inhibitor binds to an allosteric site, i.e. A site different from the active site where the substrate binds. The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. Many regulatory enzymes also display allosteric activation, and a single effector often acts in opposite directions on opposing reactions:
From microbenotes.com
Allosteric Inhibition Mechanism, Cooperativity, Examples In Mixed Inhibition The Allosteric Effect Effects Allosteric interactions occur when the binding of a ligand (not necessarily a substrate) to a. For example, in many organisms. An allosteric inhibitor decreases activity by binding to an. The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. A site different from the active site where the substrate binds. If. In Mixed Inhibition The Allosteric Effect Effects.
From www.slideserve.com
PPT to class of Enzyme and Inhibition PowerPoint Presentation ID4028909 In Mixed Inhibition The Allosteric Effect Effects The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. An allosteric inhibitor decreases activity by binding to an. If s and i bound to different sites, and s bound to e and produced a conformational change in e such that i could not bind (and vice. Many regulatory enzymes also. In Mixed Inhibition The Allosteric Effect Effects.
From www.slideserve.com
PPT Chapter 6 Enzymes PowerPoint Presentation, free download ID5869201 In Mixed Inhibition The Allosteric Effect Effects Many regulatory enzymes also display allosteric activation, and a single effector often acts in opposite directions on opposing reactions: If s and i bound to different sites, and s bound to e and produced a conformational change in e such that i could not bind (and vice. In mixed inhibition, the inhibitor binds to an allosteric site, i.e. A site. In Mixed Inhibition The Allosteric Effect Effects.
From proper-cooking.info
Allosteric Inhibition Graph In Mixed Inhibition The Allosteric Effect Effects For example, in many organisms. A site different from the active site where the substrate binds. Many regulatory enzymes also display allosteric activation, and a single effector often acts in opposite directions on opposing reactions: In mixed inhibition, the inhibitor binds to an allosteric site, i.e. Allosteric interactions occur when the binding of a ligand (not necessarily a substrate) to. In Mixed Inhibition The Allosteric Effect Effects.
From www.slideserve.com
PPT Allosteric enzymes PowerPoint Presentation ID3759029 In Mixed Inhibition The Allosteric Effect Effects For example, in many organisms. The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. Many regulatory enzymes also display allosteric activation, and a single effector often acts in opposite directions on opposing reactions: In mixed inhibition, the inhibitor binds to an allosteric site, i.e. A site different from the active. In Mixed Inhibition The Allosteric Effect Effects.
From www.animalia-life.club
Mixed Inhibition Graph In Mixed Inhibition The Allosteric Effect Effects An allosteric inhibitor decreases activity by binding to an. Allosteric interactions occur when the binding of a ligand (not necessarily a substrate) to a. The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. Many regulatory enzymes also display allosteric activation, and a single effector often acts in opposite directions on. In Mixed Inhibition The Allosteric Effect Effects.
From slideplayer.com
Chapter 6&7. The Behavior of Proteins. ppt download In Mixed Inhibition The Allosteric Effect Effects For example, in many organisms. In mixed inhibition, the inhibitor binds to an allosteric site, i.e. The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. An allosteric inhibitor decreases activity by binding to an. Many regulatory enzymes also display allosteric activation, and a single effector often acts in opposite directions. In Mixed Inhibition The Allosteric Effect Effects.
From www.youtube.com
Mixed inhibition and Non competitive inhibition YouTube In Mixed Inhibition The Allosteric Effect Effects A site different from the active site where the substrate binds. An allosteric inhibitor decreases activity by binding to an. The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. Allosteric interactions occur when the binding of a ligand (not necessarily a substrate) to a. In mixed inhibition, the inhibitor binds. In Mixed Inhibition The Allosteric Effect Effects.
From quizlet.com
Allosteric activators and inhibitors Diagram Quizlet In Mixed Inhibition The Allosteric Effect Effects Allosteric interactions occur when the binding of a ligand (not necessarily a substrate) to a. In mixed inhibition, the inhibitor binds to an allosteric site, i.e. The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. An allosteric inhibitor decreases activity by binding to an. For example, in many organisms. A. In Mixed Inhibition The Allosteric Effect Effects.
From openoregon.pressbooks.pub
Enzymes Principles of Biology In Mixed Inhibition The Allosteric Effect Effects In mixed inhibition, the inhibitor binds to an allosteric site, i.e. For example, in many organisms. If s and i bound to different sites, and s bound to e and produced a conformational change in e such that i could not bind (and vice. A site different from the active site where the substrate binds. The combined chespa and md. In Mixed Inhibition The Allosteric Effect Effects.
From www.lecturio.com
Enzyme Inhibition Concise Medical Knowledge In Mixed Inhibition The Allosteric Effect Effects An allosteric inhibitor decreases activity by binding to an. Many regulatory enzymes also display allosteric activation, and a single effector often acts in opposite directions on opposing reactions: Allosteric interactions occur when the binding of a ligand (not necessarily a substrate) to a. The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to. In Mixed Inhibition The Allosteric Effect Effects.
From answercatalexis.z21.web.core.windows.net
What Is Allosteric Inhibition In Mixed Inhibition The Allosteric Effect Effects The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. If s and i bound to different sites, and s bound to e and produced a conformational change in e such that i could not bind (and vice. For example, in many organisms. In mixed inhibition, the inhibitor binds to an. In Mixed Inhibition The Allosteric Effect Effects.
From www.animalia-life.club
Mixed Inhibition Graph In Mixed Inhibition The Allosteric Effect Effects An allosteric inhibitor decreases activity by binding to an. A site different from the active site where the substrate binds. If s and i bound to different sites, and s bound to e and produced a conformational change in e such that i could not bind (and vice. Many regulatory enzymes also display allosteric activation, and a single effector often. In Mixed Inhibition The Allosteric Effect Effects.
From www.lecturio.com
Enzyme Inhibition Concise Medical Knowledge In Mixed Inhibition The Allosteric Effect Effects For example, in many organisms. In mixed inhibition, the inhibitor binds to an allosteric site, i.e. Allosteric interactions occur when the binding of a ligand (not necessarily a substrate) to a. The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. Many regulatory enzymes also display allosteric activation, and a single. In Mixed Inhibition The Allosteric Effect Effects.
From slideplayer.com
Part 2 INHIBITION ALLOSTERIC REGULATION FEEDBACK INHIBITION ppt download In Mixed Inhibition The Allosteric Effect Effects In mixed inhibition, the inhibitor binds to an allosteric site, i.e. Many regulatory enzymes also display allosteric activation, and a single effector often acts in opposite directions on opposing reactions: For example, in many organisms. Allosteric interactions occur when the binding of a ligand (not necessarily a substrate) to a. A site different from the active site where the substrate. In Mixed Inhibition The Allosteric Effect Effects.
From www.slideserve.com
PPT Allosteric enzymes PowerPoint Presentation, free download ID3759029 In Mixed Inhibition The Allosteric Effect Effects The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. Many regulatory enzymes also display allosteric activation, and a single effector often acts in opposite directions on opposing reactions: Allosteric interactions occur when the binding of a ligand (not necessarily a substrate) to a. A site different from the active site. In Mixed Inhibition The Allosteric Effect Effects.
From drawittoknowit.com
Biochemistry Hemoglobin Allosteric Effects ditki medical & biological sciences In Mixed Inhibition The Allosteric Effect Effects In mixed inhibition, the inhibitor binds to an allosteric site, i.e. A site different from the active site where the substrate binds. The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. If s and i bound to different sites, and s bound to e and produced a conformational change in. In Mixed Inhibition The Allosteric Effect Effects.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID305372 In Mixed Inhibition The Allosteric Effect Effects An allosteric inhibitor decreases activity by binding to an. In mixed inhibition, the inhibitor binds to an allosteric site, i.e. A site different from the active site where the substrate binds. The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. Many regulatory enzymes also display allosteric activation, and a single. In Mixed Inhibition The Allosteric Effect Effects.
From www.researchgate.net
Differential effects of the allosteric inhibitor 27 and the ATPsite... Download Scientific In Mixed Inhibition The Allosteric Effect Effects An allosteric inhibitor decreases activity by binding to an. The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. Many regulatory enzymes also display allosteric activation, and a single effector often acts in opposite directions on opposing reactions: A site different from the active site where the substrate binds. In mixed. In Mixed Inhibition The Allosteric Effect Effects.
From microbenotes.com
Allosteric Inhibition Mechanism, Cooperativity, Examples In Mixed Inhibition The Allosteric Effect Effects Many regulatory enzymes also display allosteric activation, and a single effector often acts in opposite directions on opposing reactions: An allosteric inhibitor decreases activity by binding to an. If s and i bound to different sites, and s bound to e and produced a conformational change in e such that i could not bind (and vice. In mixed inhibition, the. In Mixed Inhibition The Allosteric Effect Effects.
From www.slideserve.com
PPT Allosteric enzymes PowerPoint Presentation, free download ID3759029 In Mixed Inhibition The Allosteric Effect Effects An allosteric inhibitor decreases activity by binding to an. For example, in many organisms. A site different from the active site where the substrate binds. The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. Many regulatory enzymes also display allosteric activation, and a single effector often acts in opposite directions. In Mixed Inhibition The Allosteric Effect Effects.
From www.slideserve.com
PPT Allosteric enzymes PowerPoint Presentation ID3759029 In Mixed Inhibition The Allosteric Effect Effects The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. An allosteric inhibitor decreases activity by binding to an. A site different from the active site where the substrate binds. Many regulatory enzymes also display allosteric activation, and a single effector often acts in opposite directions on opposing reactions: In mixed. In Mixed Inhibition The Allosteric Effect Effects.
From www.slideserve.com
PPT Allosteric regulation of enzyme activity PowerPoint Presentation ID3695017 In Mixed Inhibition The Allosteric Effect Effects A site different from the active site where the substrate binds. Many regulatory enzymes also display allosteric activation, and a single effector often acts in opposite directions on opposing reactions: For example, in many organisms. The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. An allosteric inhibitor decreases activity by. In Mixed Inhibition The Allosteric Effect Effects.
From www.slideserve.com
PPT LECTURE 2 ENZYME PowerPoint Presentation, free download ID5086606 In Mixed Inhibition The Allosteric Effect Effects In mixed inhibition, the inhibitor binds to an allosteric site, i.e. The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. An allosteric inhibitor decreases activity by binding to an. Many regulatory enzymes also display allosteric activation, and a single effector often acts in opposite directions on opposing reactions: For example,. In Mixed Inhibition The Allosteric Effect Effects.
From ar.inspiredpencil.com
Mixed Inhibition Michaelis Menten In Mixed Inhibition The Allosteric Effect Effects Many regulatory enzymes also display allosteric activation, and a single effector often acts in opposite directions on opposing reactions: A site different from the active site where the substrate binds. In mixed inhibition, the inhibitor binds to an allosteric site, i.e. For example, in many organisms. Allosteric interactions occur when the binding of a ligand (not necessarily a substrate) to. In Mixed Inhibition The Allosteric Effect Effects.
From www.vedantu.com
Why is allosteric inhibition called feedback inhibition? Explain. In Mixed Inhibition The Allosteric Effect Effects For example, in many organisms. An allosteric inhibitor decreases activity by binding to an. In mixed inhibition, the inhibitor binds to an allosteric site, i.e. The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. If s and i bound to different sites, and s bound to e and produced a. In Mixed Inhibition The Allosteric Effect Effects.
From microbenotes.com
Allosteric Inhibition Mechanism, Cooperativity, Examples In Mixed Inhibition The Allosteric Effect Effects For example, in many organisms. Allosteric interactions occur when the binding of a ligand (not necessarily a substrate) to a. In mixed inhibition, the inhibitor binds to an allosteric site, i.e. The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. If s and i bound to different sites, and s. In Mixed Inhibition The Allosteric Effect Effects.
From teachmephysiology.com
Enzyme Inhibition Types of Inhibition Allosteric Regulation TeachMePhysiology In Mixed Inhibition The Allosteric Effect Effects A site different from the active site where the substrate binds. The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. If s and i bound to different sites, and s bound to e and produced a conformational change in e such that i could not bind (and vice. In mixed. In Mixed Inhibition The Allosteric Effect Effects.
From www.slideserve.com
PPT Biochemistry PowerPoint Presentation, free download ID5755720 In Mixed Inhibition The Allosteric Effect Effects A site different from the active site where the substrate binds. If s and i bound to different sites, and s bound to e and produced a conformational change in e such that i could not bind (and vice. The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. In mixed. In Mixed Inhibition The Allosteric Effect Effects.
From www.slideserve.com
PPT Enzyme Inhibition PowerPoint Presentation, free download ID2697410 In Mixed Inhibition The Allosteric Effect Effects The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. An allosteric inhibitor decreases activity by binding to an. If s and i bound to different sites, and s bound to e and produced a conformational change in e such that i could not bind (and vice. Many regulatory enzymes also. In Mixed Inhibition The Allosteric Effect Effects.
From www.animalia-life.club
Mixed Inhibition Graph In Mixed Inhibition The Allosteric Effect Effects A site different from the active site where the substrate binds. The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. Many regulatory enzymes also display allosteric activation, and a single effector often acts in opposite directions on opposing reactions: An allosteric inhibitor decreases activity by binding to an. If s. In Mixed Inhibition The Allosteric Effect Effects.
From www.slideserve.com
PPT Chapter 6 An Introduction To Metabolism PowerPoint Presentation, free download ID5635865 In Mixed Inhibition The Allosteric Effect Effects For example, in many organisms. Many regulatory enzymes also display allosteric activation, and a single effector often acts in opposite directions on opposing reactions: In mixed inhibition, the inhibitor binds to an allosteric site, i.e. A site different from the active site where the substrate binds. An allosteric inhibitor decreases activity by binding to an. If s and i bound. In Mixed Inhibition The Allosteric Effect Effects.
From www.medschoolcoach.com
Lineweaver Burk Plots MCAT Biochemistry MedSchoolCoach In Mixed Inhibition The Allosteric Effect Effects For example, in many organisms. Many regulatory enzymes also display allosteric activation, and a single effector often acts in opposite directions on opposing reactions: The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. If s and i bound to different sites, and s bound to e and produced a conformational. In Mixed Inhibition The Allosteric Effect Effects.
From hra.animalia-life.club
Mixed Inhibition In Mixed Inhibition The Allosteric Effect Effects If s and i bound to different sites, and s bound to e and produced a conformational change in e such that i could not bind (and vice. The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. Allosteric interactions occur when the binding of a ligand (not necessarily a substrate). In Mixed Inhibition The Allosteric Effect Effects.
From courses.lumenlearning.com
Enzymes OpenStax Biology 2e In Mixed Inhibition The Allosteric Effect Effects The combined chespa and md results show that sampling a mixed intermediate state allows the inhibitor to preserve high. An allosteric inhibitor decreases activity by binding to an. If s and i bound to different sites, and s bound to e and produced a conformational change in e such that i could not bind (and vice. For example, in many. In Mixed Inhibition The Allosteric Effect Effects.