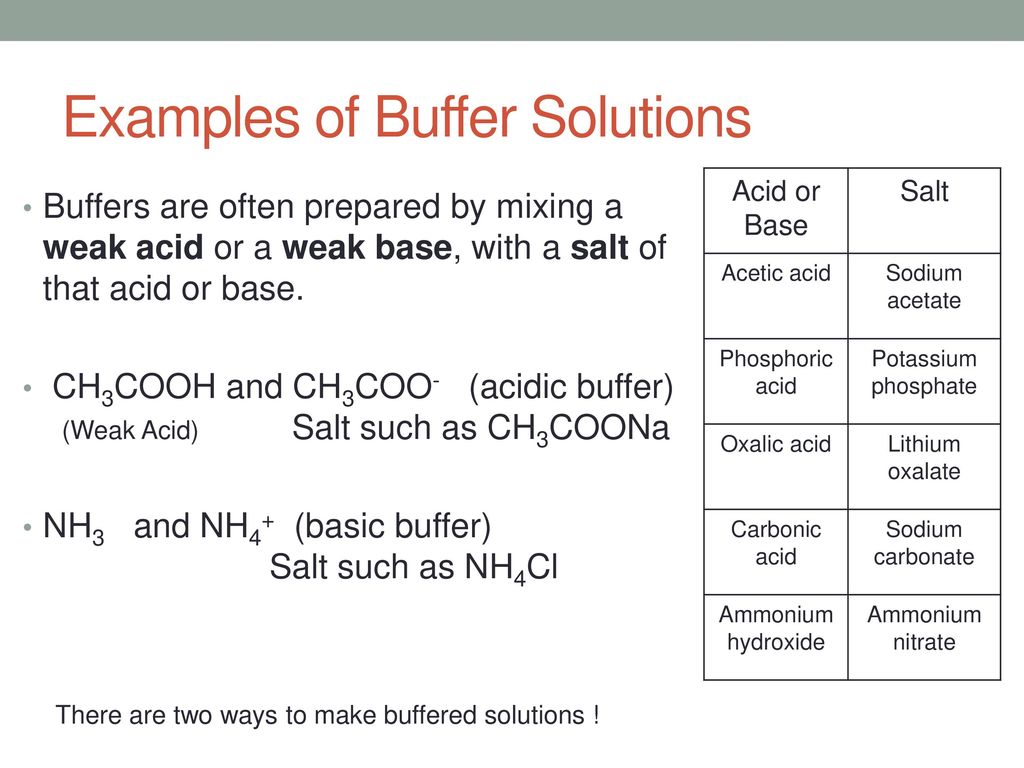
Buffer Solution Example . for example, a buffer can be composed of dissolved acetic acid (hc 2 h 3 o 2, a weak acid) and sodium acetate (nac 2 h 3 o 2, a salt derived from that acid). this page describes simple acidic and alkaline buffer solutions and explains how they work. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. It is able to neutralize. A buffer solution is one which. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl, a salt derived from that base). This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. What is a buffer solution? a buffer is a solution that can resist ph change upon the addition of an acidic or basic components.
from ar.inspiredpencil.com
for example, a buffer can be composed of dissolved acetic acid (hc 2 h 3 o 2, a weak acid) and sodium acetate (nac 2 h 3 o 2, a salt derived from that acid). It is able to neutralize. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl, a salt derived from that base). This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. this page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer solution is one which. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. What is a buffer solution?
Buffer Chemistry Example
Buffer Solution Example Another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl, a salt derived from that base). A buffer solution is one which. It is able to neutralize. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl, a salt derived from that base). this page describes simple acidic and alkaline buffer solutions and explains how they work. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. What is a buffer solution? for example, a buffer can be composed of dissolved acetic acid (hc 2 h 3 o 2, a weak acid) and sodium acetate (nac 2 h 3 o 2, a salt derived from that acid). a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is.
From www.youtube.com
Buffer Solutions YouTube Buffer Solution Example a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize. What is a buffer solution? a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one which. . Buffer Solution Example.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID6787517 Buffer Solution Example It is able to neutralize. this page describes simple acidic and alkaline buffer solutions and explains how they work. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a buffer is a solution that can resist ph change upon the addition of an acidic. Buffer Solution Example.
From skc13chem.blogspot.com
SKC year 13 Chemistry Buffer solutions Buffer Solution Example a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. for example, a buffer can be composed of dissolved acetic acid (hc 2 h 3 o 2, a weak acid) and sodium acetate (nac 2 h 3 o 2, a salt derived from that. Buffer Solution Example.
From www.sliderbase.com
Buffers Presentation Chemistry Buffer Solution Example a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. It is able to neutralize. A buffer solution is one which. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. . Buffer Solution Example.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Solution Example Another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl, a salt derived from that base). this page describes simple acidic and alkaline buffer solutions and explains how they work. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. a buffer. Buffer Solution Example.
From ar.inspiredpencil.com
Buffer Chemistry Example Buffer Solution Example for example, a buffer can be composed of dissolved acetic acid (hc 2 h 3 o 2, a weak acid) and sodium acetate (nac 2 h 3 o 2, a salt derived from that acid). It is able to neutralize. this page describes simple acidic and alkaline buffer solutions and explains how they work. Another example of a. Buffer Solution Example.
From www.slideshare.net
2012 topic 18 2 buffer solutions Buffer Solution Example A buffer solution is one which. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. this page describes simple acidic and alkaline buffer solutions and explains how they work. What is a buffer solution? a solution whose ph is not altered to any great. Buffer Solution Example.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Buffer Solution Example A buffer solution is one which. this page describes simple acidic and alkaline buffer solutions and explains how they work. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize. What is a buffer solution? for example, a buffer can. Buffer Solution Example.
From www.coursehero.com
[Solved] 1. Explain what is in a buffer. Discuss the function of a Buffer Solution Example a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. a buffer is a solution that maintains the stability of a system’s ph level. Buffer Solution Example.
From www.pinterest.com
Buffer solutions Buffer solution, Chemistry lessons, Fun learning Buffer Solution Example a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. this page describes simple acidic and alkaline buffer solutions and explains how they work. It is able to neutralize. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial.. Buffer Solution Example.
From studylib.net
Buffer Solutions Buffer Solution Example for example, a buffer can be composed of dissolved acetic acid (hc 2 h 3 o 2, a weak acid) and sodium acetate (nac 2 h 3 o 2, a salt derived from that acid). This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. It is able to neutralize. a buffer is. Buffer Solution Example.
From www.youtube.com
CALCULATION pH OF BUFFER SOLUTION EXAMPLE 2 YouTube Buffer Solution Example a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. for example, a buffer can be composed of dissolved acetic acid (hc 2 h. Buffer Solution Example.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Buffer Solution Example Another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl, a salt derived from that base). a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a solution whose ph is not altered to any. Buffer Solution Example.
From www.youtube.com
Calculating pH of buffer solution with Strong Acid YouTube Buffer Solution Example a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. for example, a buffer can be composed of dissolved acetic acid (hc 2 h 3 o 2, a weak acid) and sodium acetate (nac 2 h 3 o 2, a salt derived from that acid). This characteristic makes buffers. Buffer Solution Example.
From design.udlvirtual.edu.pe
What Is The Ph Of A Buffer Solution Design Talk Buffer Solution Example A buffer solution is one which. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. for example, a buffer can be composed of dissolved acetic. Buffer Solution Example.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Buffer Solution Example a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. Another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl, a salt derived from that base). for example, a buffer can be composed. Buffer Solution Example.
From skc13chem.blogspot.com
SKC year 13 Chemistry Buffer solutions Buffer Solution Example a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. for example, a buffer can be composed of dissolved acetic acid (hc 2 h 3 o 2, a weak acid) and sodium acetate (nac 2 h 3 o 2, a salt derived from that acid). . Buffer Solution Example.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your Buffer Solution Example It is able to neutralize. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. this page describes simple acidic and alkaline buffer solutions and explains how they work. for example, a buffer can be composed of dissolved acetic acid (hc 2 h 3 o 2, a weak acid) and sodium acetate (nac. Buffer Solution Example.
From thechemistrynotes.com
Buffer Solution Types, Properties, and Uses Buffer Solution Example for example, a buffer can be composed of dissolved acetic acid (hc 2 h 3 o 2, a weak acid) and sodium acetate (nac 2 h 3 o 2, a salt derived from that acid). What is a buffer solution? a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities. Buffer Solution Example.
From www.tes.com
Buffer Solutions 3 (A Level Chemistry) Teaching Resources Buffer Solution Example What is a buffer solution? this page describes simple acidic and alkaline buffer solutions and explains how they work. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. for example, a buffer. Buffer Solution Example.
From www.pinterest.co.uk
Buffer Easy Science Chemistry education, Study chemistry, Chemistry Buffer Solution Example a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. this page describes simple acidic and alkaline buffer solutions and explains how they work. a. Buffer Solution Example.
From sciencenotes.org
Buffer Definition and Examples in Chemistry Buffer Solution Example This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. this page describes simple acidic and alkaline buffer solutions and explains how they work. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. for example, a buffer can be composed of dissolved. Buffer Solution Example.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its Buffer Solution Example a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize. this page describes simple acidic and alkaline buffer solutions and explains how they work. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. for example, a buffer. Buffer Solution Example.
From www.ck12.org
Buffer Solutions Example 1 ( Video ) Chemistry CK12 Foundation Buffer Solution Example It is able to neutralize. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. for example, a buffer can be composed of dissolved acetic acid (hc 2 h 3 o 2, a weak acid) and sodium acetate (nac 2 h 3 o 2, a salt derived from that. Buffer Solution Example.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Buffer Solution Example a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. this page describes simple acidic and alkaline buffer solutions and explains how they work. Another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl, a salt derived from. Buffer Solution Example.
From www.slideshare.net
Lect w9 152 buffers and ksp_alg Buffer Solution Example a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Another example of a buffer is a solution containing ammonia (nh 3,. Buffer Solution Example.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Buffer Solution Example this page describes simple acidic and alkaline buffer solutions and explains how they work. What is a buffer solution? a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of. Buffer Solution Example.
From general.chemistrysteps.com
Buffer Solutions Chemistry Steps Buffer Solution Example A buffer solution is one which. It is able to neutralize. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Another example of a buffer is a solution containing ammonia (nh. Buffer Solution Example.
From www.slideshare.net
2012 topic 18 2 buffer solutions Buffer Solution Example for example, a buffer can be composed of dissolved acetic acid (hc 2 h 3 o 2, a weak acid) and sodium acetate (nac 2 h 3 o 2, a salt derived from that acid). a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. What is a buffer. Buffer Solution Example.
From www.slideserve.com
PPT Chem. Concepts Buffers PowerPoint Presentation, free download Buffer Solution Example this page describes simple acidic and alkaline buffer solutions and explains how they work. What is a buffer solution? It is able to neutralize. Another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl, a salt derived from that base). a buffer is a solution that can. Buffer Solution Example.
From cjarquin.medium.com
Aerogel Buffer Time. Week 10 Reducing Property… by Carlos Manuel Buffer Solution Example this page describes simple acidic and alkaline buffer solutions and explains how they work. for example, a buffer can be composed of dissolved acetic acid (hc 2 h 3 o 2, a weak acid) and sodium acetate (nac 2 h 3 o 2, a salt derived from that acid). a solution whose ph is not altered to. Buffer Solution Example.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Buffer Solution Example A buffer solution is one which. What is a buffer solution? a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Another example of a buffer is a solution containing ammonia (nh 3, a weak. Buffer Solution Example.
From www.youtube.com
Acidic and Basic buffer Identification and Preparation Tips and Tricks Buffer Solution Example a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution is one which. Another example of a buffer is a solution containing ammonia (nh 3, a weak base) and ammonium chloride (nh 4 cl, a salt derived from that base). a buffer is. Buffer Solution Example.
From fr.slideshare.net
Buffer solution Buffer Solution Example a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. for example, a buffer can be composed of dissolved acetic acid (hc 2 h 3 o 2, a weak acid) and sodium acetate (nac 2 h 3 o 2, a salt derived from that acid). A. Buffer Solution Example.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your Buffer Solution Example a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. this page describes simple acidic and alkaline buffer solutions and explains how they work. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. It is able to neutralize.. Buffer Solution Example.