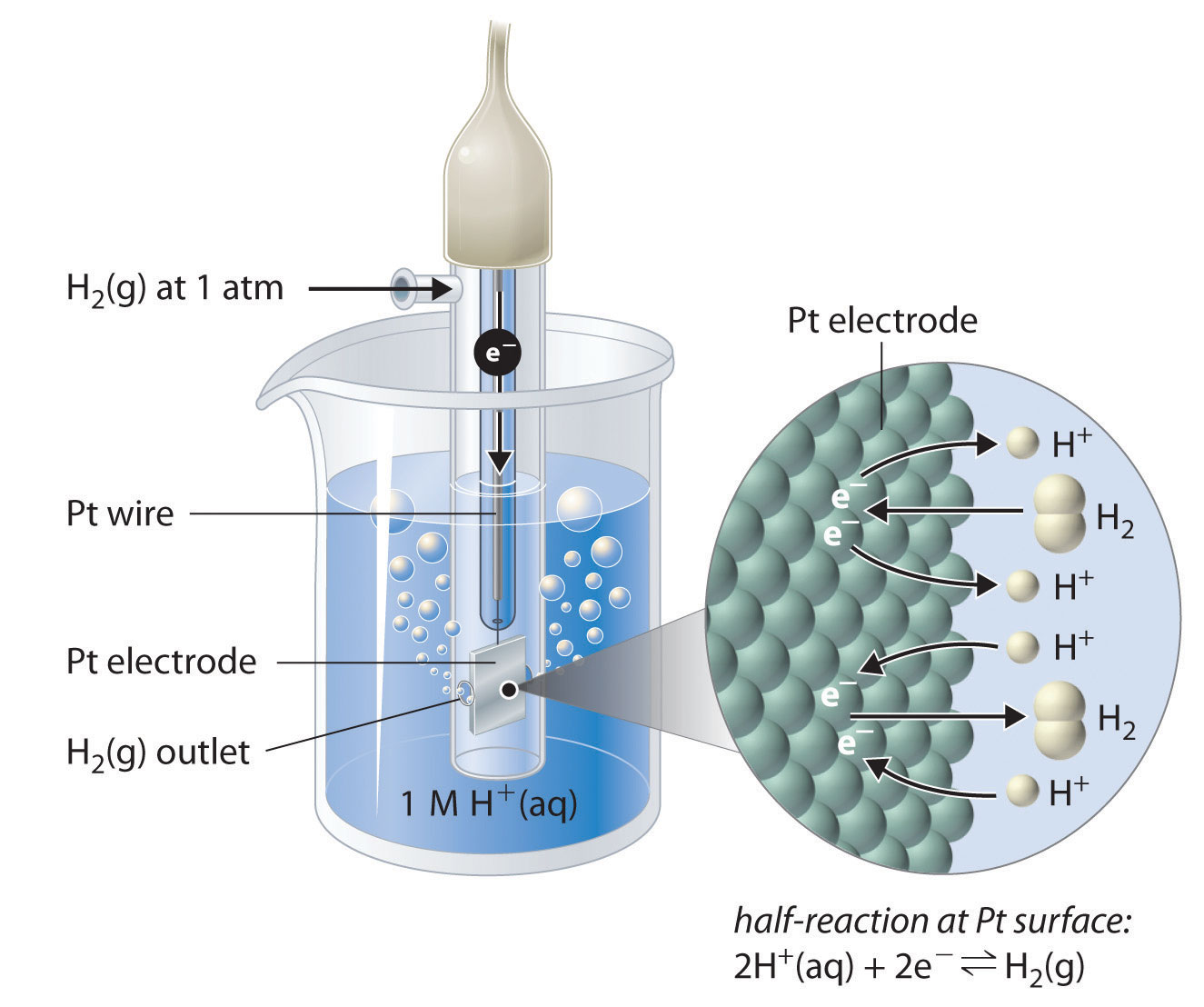
Standard Hydrogen Electrode Notation . This is because it acts as a reference for comparison with any other electrode. the standard hydrogen electrode. Examples of using the standard hydrogen electrode to determine reduction potentials are given. The standard hydrogen electrode looks like this: The structure of the standard hydrogen electrode is described. in general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. the standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. For any half reaction, we can measure the potential (free energy) by comparing it to a standard reaction.
from saylordotorg.github.io
The structure of the standard hydrogen electrode is described. the standard hydrogen electrode. This is because it acts as a reference for comparison with any other electrode. For any half reaction, we can measure the potential (free energy) by comparing it to a standard reaction. the standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Examples of using the standard hydrogen electrode to determine reduction potentials are given. The standard hydrogen electrode looks like this: in general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be.
Electrochemistry
Standard Hydrogen Electrode Notation This is because it acts as a reference for comparison with any other electrode. For any half reaction, we can measure the potential (free energy) by comparing it to a standard reaction. the standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The standard hydrogen electrode looks like this: This is because it acts as a reference for comparison with any other electrode. in general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. The structure of the standard hydrogen electrode is described. the standard hydrogen electrode. Examples of using the standard hydrogen electrode to determine reduction potentials are given.
From dxomtlfzj.blob.core.windows.net
Standard Hydrogen Electrode Conventional Representation at Bobby Standard Hydrogen Electrode Notation This is because it acts as a reference for comparison with any other electrode. The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to determine reduction potentials are given. For any half reaction, we can measure the potential (free energy) by comparing it to a standard reaction. the standard hydrogen electrode. The. Standard Hydrogen Electrode Notation.
From www.youtube.com
standard hydrogen electrode (with examples) YouTube Standard Hydrogen Electrode Notation For any half reaction, we can measure the potential (free energy) by comparing it to a standard reaction. in general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. the standard hydrogen electrode. The standard hydrogen electrode looks like this: the standard hydrogen electrode is. Standard Hydrogen Electrode Notation.
From www.shutterstock.com
Diagramme standard d'électrode à hydrogène. Illustration image Standard Hydrogen Electrode Notation the standard hydrogen electrode. the standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. This is because it acts as a reference for comparison with any other electrode. Examples of using the standard hydrogen electrode to determine reduction potentials are given. For any half. Standard Hydrogen Electrode Notation.
From exomimdmo.blob.core.windows.net
Representation Of Standard Hydrogen Electrode at Rona Loomis blog Standard Hydrogen Electrode Notation For any half reaction, we can measure the potential (free energy) by comparing it to a standard reaction. the standard hydrogen electrode. The standard hydrogen electrode looks like this: Examples of using the standard hydrogen electrode to determine reduction potentials are given. This is because it acts as a reference for comparison with any other electrode. the standard. Standard Hydrogen Electrode Notation.
From www.youtube.com
The Standard hydrogen electrode (SHE) YouTube Standard Hydrogen Electrode Notation the standard hydrogen electrode. the standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. This is because it acts as a reference for comparison with any other electrode. The standard hydrogen electrode looks like this: Examples of using the standard hydrogen electrode to determine. Standard Hydrogen Electrode Notation.
From www.savemyexams.com
The Hydrogen Electrode (HL) HL IB Chemistry Revision Notes 2025 Standard Hydrogen Electrode Notation the standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Examples of using the standard hydrogen electrode to determine reduction potentials are given. This is because it acts as a reference for comparison with any other electrode. in general, if a galvanic cell is. Standard Hydrogen Electrode Notation.
From www.youtube.com
Electrochemistry 5 Standard hydrogen electrode YouTube Standard Hydrogen Electrode Notation The structure of the standard hydrogen electrode is described. the standard hydrogen electrode. This is because it acts as a reference for comparison with any other electrode. Examples of using the standard hydrogen electrode to determine reduction potentials are given. The standard hydrogen electrode looks like this: the standard hydrogen electrode is often abbreviated to she, and its. Standard Hydrogen Electrode Notation.
From saylordotorg.github.io
Standard Potentials Standard Hydrogen Electrode Notation The structure of the standard hydrogen electrode is described. This is because it acts as a reference for comparison with any other electrode. The standard hydrogen electrode looks like this: in general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. For any half reaction, we can. Standard Hydrogen Electrode Notation.
From saylordotorg.github.io
Electrochemistry Standard Hydrogen Electrode Notation the standard hydrogen electrode. in general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. Examples of using the standard hydrogen electrode to determine reduction potentials are given. This is because it acts as a reference for comparison with any other electrode. the standard hydrogen. Standard Hydrogen Electrode Notation.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Standard Hydrogen Electrode Notation Examples of using the standard hydrogen electrode to determine reduction potentials are given. in general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. the standard hydrogen electrode. For any half reaction, we can measure the potential (free energy) by comparing it to a standard reaction.. Standard Hydrogen Electrode Notation.
From monomole.com
Standard hydrogen electrode Mono Mole Standard Hydrogen Electrode Notation This is because it acts as a reference for comparison with any other electrode. Examples of using the standard hydrogen electrode to determine reduction potentials are given. the standard hydrogen electrode. For any half reaction, we can measure the potential (free energy) by comparing it to a standard reaction. in general, if a galvanic cell is connected to. Standard Hydrogen Electrode Notation.
From www.youtube.com
Standard Hydrogen Electrode Demonstration YouTube Standard Hydrogen Electrode Notation in general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. This is because it acts as a reference for comparison with any other electrode. For any half reaction, we can measure the potential (free energy) by comparing it to a standard reaction. the standard hydrogen. Standard Hydrogen Electrode Notation.
From www.youtube.com
Standard hydrogen Electrode, Normal hydrogen Electrode Standard Hydrogen Electrode Notation This is because it acts as a reference for comparison with any other electrode. the standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. the standard hydrogen electrode. The structure of the standard hydrogen electrode is described. in general, if a galvanic cell. Standard Hydrogen Electrode Notation.
From mmerevise.co.uk
Electrochemical Cells MME Standard Hydrogen Electrode Notation The standard hydrogen electrode looks like this: This is because it acts as a reference for comparison with any other electrode. The structure of the standard hydrogen electrode is described. the standard hydrogen electrode. For any half reaction, we can measure the potential (free energy) by comparing it to a standard reaction. in general, if a galvanic cell. Standard Hydrogen Electrode Notation.
From scienceinfo.com
What is Standard Hydrogen Electrode? Standard Hydrogen Electrode Notation The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to determine reduction potentials are given. This is because it acts as a reference for comparison with any other electrode. The standard hydrogen electrode looks like this: For any half reaction, we can measure the potential (free energy) by comparing it to a standard. Standard Hydrogen Electrode Notation.
From exoyfxrvd.blob.core.windows.net
Standard Hydrogen Electrode Nernst Equation at Silva Upchurch blog Standard Hydrogen Electrode Notation in general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. the standard hydrogen electrode. Examples of using the standard hydrogen electrode to determine reduction potentials are given. The structure of the standard hydrogen electrode is described. For any half reaction, we can measure the potential. Standard Hydrogen Electrode Notation.
From www.slideserve.com
PPT Fundamentals of Electrochemistry PowerPoint Presentation, free Standard Hydrogen Electrode Notation The standard hydrogen electrode looks like this: For any half reaction, we can measure the potential (free energy) by comparing it to a standard reaction. in general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. the standard hydrogen electrode. This is because it acts as. Standard Hydrogen Electrode Notation.
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free Standard Hydrogen Electrode Notation For any half reaction, we can measure the potential (free energy) by comparing it to a standard reaction. in general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. Examples of using the standard hydrogen electrode to determine reduction potentials are given. the standard hydrogen electrode. Standard Hydrogen Electrode Notation.
From exoyfxrvd.blob.core.windows.net
Standard Hydrogen Electrode Nernst Equation at Silva Upchurch blog Standard Hydrogen Electrode Notation This is because it acts as a reference for comparison with any other electrode. the standard hydrogen electrode. Examples of using the standard hydrogen electrode to determine reduction potentials are given. the standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. For any half. Standard Hydrogen Electrode Notation.
From www.youtube.com
Standard Hydrogen Electrode (SHE)/ Primary reference electrode Gas Standard Hydrogen Electrode Notation in general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. This is because it acts as a reference for comparison with any other electrode. The structure of the standard hydrogen electrode is described. the standard hydrogen electrode. The standard hydrogen electrode looks like this: Examples. Standard Hydrogen Electrode Notation.
From sulekharanirxiichemistry.blogspot.com
SULEKHA Class XII Chemistry STANDARD HYDROGEN ELECTRODE Standard Hydrogen Electrode Notation Examples of using the standard hydrogen electrode to determine reduction potentials are given. For any half reaction, we can measure the potential (free energy) by comparing it to a standard reaction. This is because it acts as a reference for comparison with any other electrode. The standard hydrogen electrode looks like this: the standard hydrogen electrode is often abbreviated. Standard Hydrogen Electrode Notation.
From www.vrogue.co
Hydrogen Standard Hydrogen Electrode vrogue.co Standard Hydrogen Electrode Notation The standard hydrogen electrode looks like this: in general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. The structure of the standard hydrogen electrode is described. the standard hydrogen electrode. the standard hydrogen electrode is often abbreviated to she, and its standard electrode potential. Standard Hydrogen Electrode Notation.
From cider.uoregon.edu
Standard Hydrogen Electrode Demonstration and AACT Simulation CIDER Standard Hydrogen Electrode Notation in general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. The standard hydrogen electrode looks like this: the standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Examples of using the. Standard Hydrogen Electrode Notation.
From chemistnotes.com
Standard hydrogen electrode(SHE) Definition, diagram, application Standard Hydrogen Electrode Notation Examples of using the standard hydrogen electrode to determine reduction potentials are given. The structure of the standard hydrogen electrode is described. the standard hydrogen electrode. in general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. This is because it acts as a reference for. Standard Hydrogen Electrode Notation.
From twitter.com
Digital Kemistry Free Online Chemistry Learning on Twitter "Do you Standard Hydrogen Electrode Notation the standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The standard hydrogen electrode looks like this: Examples of using the standard hydrogen electrode to determine reduction potentials are given. The structure of the standard hydrogen electrode is described. in general, if a galvanic. Standard Hydrogen Electrode Notation.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID4435141 Standard Hydrogen Electrode Notation the standard hydrogen electrode. For any half reaction, we can measure the potential (free energy) by comparing it to a standard reaction. in general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. the standard hydrogen electrode is often abbreviated to she, and its standard. Standard Hydrogen Electrode Notation.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Standard Hydrogen Electrode Notation The standard hydrogen electrode looks like this: the standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Examples of using the standard hydrogen electrode to determine reduction potentials are given. For any half reaction, we can measure the potential (free energy) by comparing it to. Standard Hydrogen Electrode Notation.
From www.slideserve.com
PPT Electroanalysis PowerPoint Presentation, free download ID6733376 Standard Hydrogen Electrode Notation in general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. The standard hydrogen electrode looks like this: For any half reaction, we can measure the potential (free energy) by comparing it to a standard reaction. This is because it acts as a reference for comparison with. Standard Hydrogen Electrode Notation.
From dxomtlfzj.blob.core.windows.net
Standard Hydrogen Electrode Conventional Representation at Bobby Standard Hydrogen Electrode Notation For any half reaction, we can measure the potential (free energy) by comparing it to a standard reaction. the standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to. Standard Hydrogen Electrode Notation.
From www.researchgate.net
Absolute and relative (respect to Standard Hydrogen Electrode, SHE Standard Hydrogen Electrode Notation in general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. This is because it acts as a reference for comparison with any other electrode. The standard hydrogen electrode looks like this: the standard hydrogen electrode is often abbreviated to she, and its standard electrode potential. Standard Hydrogen Electrode Notation.
From www.youtube.com
Standard hydrogen electrode ( SHE ) , EC CELL, NERNST EQUATION BY Standard Hydrogen Electrode Notation For any half reaction, we can measure the potential (free energy) by comparing it to a standard reaction. the standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. This is because it acts as a reference for comparison with any other electrode. the standard. Standard Hydrogen Electrode Notation.
From byjus.com
What is standard hydrogen electrode? Standard Hydrogen Electrode Notation the standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The standard hydrogen electrode looks like this: the standard hydrogen electrode. Examples of using the standard hydrogen electrode to determine reduction potentials are given. This is because it acts as a reference for comparison. Standard Hydrogen Electrode Notation.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Hydrogen Electrode Notation the standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to determine reduction potentials are given. The standard hydrogen electrode looks like this: For any half reaction, we can. Standard Hydrogen Electrode Notation.
From www.youtube.com
Define standard hydrogen electrode SHE and write the reactions that Standard Hydrogen Electrode Notation the standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Examples of using the standard hydrogen electrode to determine reduction potentials are given. For any half reaction, we can measure the potential (free energy) by comparing it to a standard reaction. This is because it. Standard Hydrogen Electrode Notation.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12 Standard Hydrogen Electrode Notation The structure of the standard hydrogen electrode is described. the standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. in general, if a galvanic cell is connected to a voltmeter, the electrode connected to the negative terminal of the meter must be. This is. Standard Hydrogen Electrode Notation.