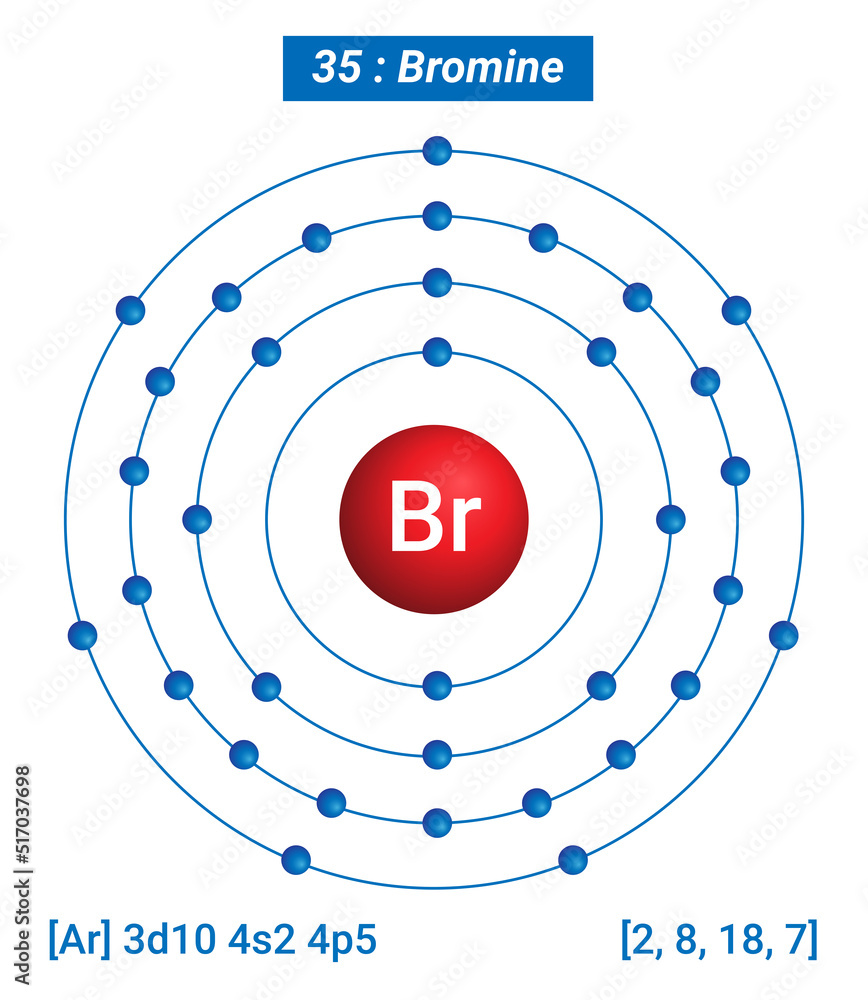
Bromine Total Electrons . The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Surrounding this nucleus are four electron shells, holding a total of 35 electrons. The bromine bohr model has a nucleus with 35 protons and 45 neutrons. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. The number of neutrons in an atom can be determined by the difference between the atomic. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. It has an atomic weight of 79.904 and a mass. It was the first element to be extracted. Bromine is a chemical element with atomic number 35 which means there are 35 protons in its nucleus. Like all halogens, it is thus one.
from stock.adobe.com
The number of neutrons in an atom can be determined by the difference between the atomic. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Surrounding this nucleus are four electron shells, holding a total of 35 electrons. Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Bromine is a chemical element with atomic number 35 which means there are 35 protons in its nucleus. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. It was the first element to be extracted. Like all halogens, it is thus one. It has an atomic weight of 79.904 and a mass.
Br Bromine Element Information Facts, Properties, Trends, Uses and
Bromine Total Electrons Bromine is a chemical element with atomic number 35 which means there are 35 protons in its nucleus. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. It was the first element to be extracted. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. The number of neutrons in an atom can be determined by the difference between the atomic. The bromine bohr model has a nucleus with 35 protons and 45 neutrons. Bromine is a chemical element with atomic number 35 which means there are 35 protons in its nucleus. Surrounding this nucleus are four electron shells, holding a total of 35 electrons. Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Like all halogens, it is thus one. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. It has an atomic weight of 79.904 and a mass.
From www.alamy.com
3d render of atom structure of bromine isolated over white background Bromine Total Electrons The bromine bohr model has a nucleus with 35 protons and 45 neutrons. Surrounding this nucleus are four electron shells, holding a total of 35 electrons. It has an atomic weight of 79.904 and a mass. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Bromine is a. Bromine Total Electrons.
From material-properties.org
Bromine Protons Neutrons Electrons Electron Configuration Bromine Total Electrons The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. The number of neutrons in an atom can be determined by the difference between the atomic. Surrounding this nucleus are four electron shells, holding a total of 35 electrons. It has an atomic. Bromine Total Electrons.
From www.alamy.com
Bromine symbol. Chemical element of the periodic table. Vector stock Bromine Total Electrons The number of neutrons in an atom can be determined by the difference between the atomic. Surrounding this nucleus are four electron shells, holding a total of 35 electrons. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. Bromine has the electron. Bromine Total Electrons.
From www.dreamstime.com
Atom of Bromine with Detailed Core and Its 35 Electrons on Black Stock Bromine Total Electrons The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. It has an atomic weight of 79.904 and a mass. The bromine bohr model has a nucleus with 35 protons and 45 neutrons. Total number of protons in the nucleus is called the. Bromine Total Electrons.
From www.istockphoto.com
Br Bromine Element Information Facts Properties Trends Uses And Bromine Total Electrons Bromine is a chemical element with atomic number 35 which means there are 35 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Surrounding this nucleus are four electron shells, holding a total of 35 electrons. Like all halogens, it is thus one. Bromine. Bromine Total Electrons.
From www.dreamstime.com
Periodic Table of Elements Bromine Stock Illustration Illustration Bromine Total Electrons Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. Bromine is a chemical element with atomic number 35 which means there. Bromine Total Electrons.
From material-properties.org
Bromine Periodic Table and Atomic Properties Bromine Total Electrons The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. Bromine is a chemical element with atomic number 35 which means there are 35 protons in its nucleus. Like all halogens, it is thus one. The bromine bohr model has a nucleus with. Bromine Total Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Total Electrons It was the first element to be extracted. It has an atomic weight of 79.904 and a mass. Bromine is a chemical element with atomic number 35 which means there are 35 protons in its nucleus. Surrounding this nucleus are four electron shells, holding a total of 35 electrons. Total number of protons in the nucleus is called the atomic. Bromine Total Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Total Electrons The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The bromine bohr model has a nucleus with 35 protons and 45 neutrons.. Bromine Total Electrons.
From www.vectorstock.com
Bromine br periodic table element Royalty Free Vector Image Bromine Total Electrons Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6. Bromine Total Electrons.
From periodictable.me
2000pxElectron_configuration_bromine.svg Dynamic Periodic Table of Bromine Total Electrons It was the first element to be extracted. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The number of neutrons in an atom can be determined by the difference between the atomic. Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in. Bromine Total Electrons.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Total Electrons The bromine bohr model has a nucleus with 35 protons and 45 neutrons. Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. It. Bromine Total Electrons.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Bromine Total Electrons Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Bromine is a chemical element with atomic number 35 which means there are 35. Bromine Total Electrons.
From www.dreamstime.com
Element of Bromine stock vector. Illustration of college 104400483 Bromine Total Electrons The number of neutrons in an atom can be determined by the difference between the atomic. Surrounding this nucleus are four electron shells, holding a total of 35 electrons. It was the first element to be extracted. It has an atomic weight of 79.904 and a mass. Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the. Bromine Total Electrons.
From www.slideserve.com
PPT Topic Chemistry Aim Explain how elements are classified in Bromine Total Electrons Surrounding this nucleus are four electron shells, holding a total of 35 electrons. Like all halogens, it is thus one. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. The bromine bohr model has a nucleus with 35 protons and 45 neutrons.. Bromine Total Electrons.
From autoctrls.com
Understanding the Bromine Electron Dot Diagram A Comprehensive Guide Bromine Total Electrons The number of neutrons in an atom can be determined by the difference between the atomic. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Surrounding this nucleus are four electron shells, holding a total of 35 electrons. Bromine is a chemical element with atomic number 35 which means. Bromine Total Electrons.
From www.alamy.com
Symbol and electron diagram for Bromine illustration Stock Vector Image Bromine Total Electrons Like all halogens, it is thus one. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Surrounding this nucleus are four electron shells, holding a total of 35 electrons. It has an atomic weight of 79.904 and a mass. Bromine has the electron configuration [ar]4s 2 3d 10. Bromine Total Electrons.
From www.alamy.com
Bromine chemical element, Sign with atomic number and atomic weight Bromine Total Electrons The bromine bohr model has a nucleus with 35 protons and 45 neutrons. The number of neutrons in an atom can be determined by the difference between the atomic. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Like all halogens, it is thus one. Surrounding this nucleus. Bromine Total Electrons.
From www.alamy.com
Bromine symbol. Chemical element of the periodic table. Vector stock Bromine Total Electrons Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and. Bromine Total Electrons.
From techschematic.com
Understanding the Electron Dot Diagram for Bromine A Visual Bromine Total Electrons Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Bromine is a chemical element with atomic number 35 which means there are 35 protons in its nucleus. It has an atomic weight of 79.904 and a mass. The number of neutrons in an atom can be determined by the. Bromine Total Electrons.
From www.alamy.com
Bromine symbol. Chemical element of the periodic table. Vector stock Bromine Total Electrons Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. The number of neutrons in an atom can be determined by the. Bromine Total Electrons.
From periodictable.me
How Do We Find The Electron Configuration For Bromine Dynamic Bromine Total Electrons Like all halogens, it is thus one. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Surrounding this nucleus are four electron shells, holding a total of 35 electrons. It has an atomic weight of 79.904 and a mass. The number of neutrons in an atom can be. Bromine Total Electrons.
From www.alamy.com
Symbol and electron diagram for Bromine Stock Vector Image & Art Alamy Bromine Total Electrons The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. It was the first element to be extracted. The bromine bohr model has a nucleus with 35 protons and 45 neutrons. Surrounding this nucleus are four electron shells, holding a total of 35. Bromine Total Electrons.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Bromine Total Electrons Surrounding this nucleus are four electron shells, holding a total of 35 electrons. It has an atomic weight of 79.904 and a mass. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in. Bromine Total Electrons.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Bromine Total Electrons The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. The number of neutrons in an atom can be determined by the difference between the atomic. It has an atomic weight of 79.904 and a mass. Surrounding this nucleus are four electron shells,. Bromine Total Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Total Electrons Like all halogens, it is thus one. The number of neutrons in an atom can be determined by the difference between the atomic. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. Surrounding this nucleus are four electron shells, holding a total. Bromine Total Electrons.
From www.dreamstime.com
Bromine Atom, with Mass and Energy Levels. Stock Vector Illustration Bromine Total Electrons It has an atomic weight of 79.904 and a mass. The number of neutrons in an atom can be determined by the difference between the atomic. Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Bromine is a chemical element with atomic number. Bromine Total Electrons.
From www.alamy.com
Chemist atom of Bromine diagram Stock Vector Image & Art Alamy Bromine Total Electrons The bromine bohr model has a nucleus with 35 protons and 45 neutrons. The number of neutrons in an atom can be determined by the difference between the atomic. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2. Bromine Total Electrons.
From www.sciencephoto.com
Bromine, atomic structure Stock Image C018/3716 Science Photo Library Bromine Total Electrons The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. Surrounding this nucleus are four electron shells, holding a total of 35 electrons. Bromine is a chemical element with atomic number 35 which means there are 35 protons in its nucleus. Bromine is. Bromine Total Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Total Electrons Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and. Bromine Total Electrons.
From stock.adobe.com
Br Bromine Element Information Facts, Properties, Trends, Uses and Bromine Total Electrons The bromine bohr model has a nucleus with 35 protons and 45 neutrons. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35.. Bromine Total Electrons.
From utedzz.blogspot.com
Bromine Periodic Table Square Periodic Table Timeline Bromine Total Electrons The number of neutrons in an atom can be determined by the difference between the atomic. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. The bromine bohr model has a nucleus with 35 protons and 45 neutrons. Surrounding this nucleus are. Bromine Total Electrons.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Total Electrons The number of neutrons in an atom can be determined by the difference between the atomic. Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Like all halogens, it is thus one. Bromine is a chemical element with atomic number 35 which means. Bromine Total Electrons.
From www.dreamstime.com
Bromine Chemical 35 Element of Periodic Table. Molecule and Bromine Total Electrons Like all halogens, it is thus one. Surrounding this nucleus are four electron shells, holding a total of 35 electrons. The number of neutrons in an atom can be determined by the difference between the atomic. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Bromine has the. Bromine Total Electrons.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Bromine Total Electrons Bromine is a chemical element with atomic number 35 which means there are 35 protons in its nucleus. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. It was the first element to be extracted. The bromine bohr model has a nucleus. Bromine Total Electrons.