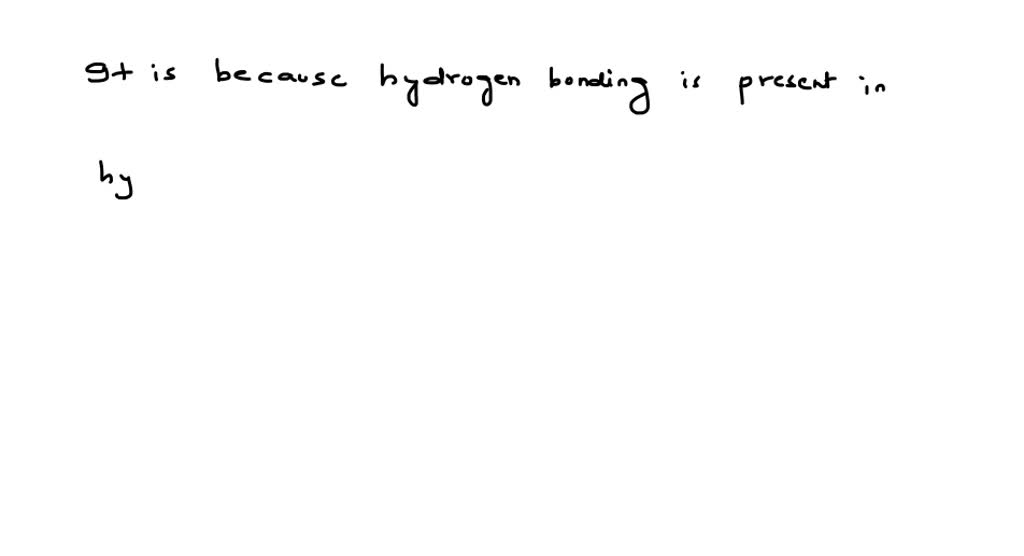Hydrogen Fluoride Is A Liquid At Room Temperature Due To . Anhydrous hf has a boiling point of 19.5°c or 67°f. Due to strong and extensive hydrogen bonding, it boils at near room temperature, which is much higher of a temperature than other hydrogen. It can exist as a colorless gas or as a fuming liquid, or it can be. Such hydrogen bonding is absent in other. Hydrogen fluoride is a colorless, fuming liquid or gas with a strong, irritating odor. When mixed with water it is called ‘hydrofluoric acid’. Hydrogen fluoride is a chemical compound that contains fluorine. Hydrogen fluoride is a liquid, and so it can be described as miscible with water. Hf is a weak acid, but its high solubility can be. As an anhydrous material, it is a colorless gas or fuming liquid. Formation of hydrogen bonding lowers energy of the system and so hf is liquid. It is usually shipped in steel cylinders as a compressed. Pure hydrogen fluoride is a colourless, fuming liquid that boils at 19.5°c. Because its boiling point is just.
from www.numerade.com
It is usually shipped in steel cylinders as a compressed. Due to strong and extensive hydrogen bonding, it boils at near room temperature, which is much higher of a temperature than other hydrogen. It can exist as a colorless gas or as a fuming liquid, or it can be. Hf is a weak acid, but its high solubility can be. Hydrogen fluoride is a colorless, fuming liquid or gas with a strong, irritating odor. Because its boiling point is just. Formation of hydrogen bonding lowers energy of the system and so hf is liquid. Hydrogen fluoride is a chemical compound that contains fluorine. Anhydrous hf has a boiling point of 19.5°c or 67°f. Pure hydrogen fluoride is a colourless, fuming liquid that boils at 19.5°c.
SOLVEDHydrogen fluoride is a liquid unlike other hydrogen halides
Hydrogen Fluoride Is A Liquid At Room Temperature Due To Hydrogen fluoride is a liquid, and so it can be described as miscible with water. Hf is a weak acid, but its high solubility can be. As an anhydrous material, it is a colorless gas or fuming liquid. Formation of hydrogen bonding lowers energy of the system and so hf is liquid. Because its boiling point is just. Hydrogen fluoride is a colorless, fuming liquid or gas with a strong, irritating odor. Hydrogen fluoride is a liquid, and so it can be described as miscible with water. Due to strong and extensive hydrogen bonding, it boils at near room temperature, which is much higher of a temperature than other hydrogen. It is usually shipped in steel cylinders as a compressed. It can exist as a colorless gas or as a fuming liquid, or it can be. Anhydrous hf has a boiling point of 19.5°c or 67°f. Hydrogen fluoride is a chemical compound that contains fluorine. Pure hydrogen fluoride is a colourless, fuming liquid that boils at 19.5°c. When mixed with water it is called ‘hydrofluoric acid’. Such hydrogen bonding is absent in other.
From www.youtube.com
What elements are liquid at room temperature? YouTube Hydrogen Fluoride Is A Liquid At Room Temperature Due To Due to strong and extensive hydrogen bonding, it boils at near room temperature, which is much higher of a temperature than other hydrogen. When mixed with water it is called ‘hydrofluoric acid’. Because its boiling point is just. Hydrogen fluoride is a colorless, fuming liquid or gas with a strong, irritating odor. Formation of hydrogen bonding lowers energy of the. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From pnghero.com
Hydrogen Fluoride Lewis Structure Covalent Bond Chemical PNG Image Hydrogen Fluoride Is A Liquid At Room Temperature Due To Anhydrous hf has a boiling point of 19.5°c or 67°f. It is usually shipped in steel cylinders as a compressed. Due to strong and extensive hydrogen bonding, it boils at near room temperature, which is much higher of a temperature than other hydrogen. Pure hydrogen fluoride is a colourless, fuming liquid that boils at 19.5°c. When mixed with water it. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From socratic.org
The liquefied hydrogen halides have the normal boiling points given Hydrogen Fluoride Is A Liquid At Room Temperature Due To Formation of hydrogen bonding lowers energy of the system and so hf is liquid. It can exist as a colorless gas or as a fuming liquid, or it can be. Pure hydrogen fluoride is a colourless, fuming liquid that boils at 19.5°c. When mixed with water it is called ‘hydrofluoric acid’. As an anhydrous material, it is a colorless gas. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From www.alamy.com
Hydrogen fluoride is a chemical compound with the formula HF. This Hydrogen Fluoride Is A Liquid At Room Temperature Due To Due to strong and extensive hydrogen bonding, it boils at near room temperature, which is much higher of a temperature than other hydrogen. When mixed with water it is called ‘hydrofluoric acid’. As an anhydrous material, it is a colorless gas or fuming liquid. It is usually shipped in steel cylinders as a compressed. Formation of hydrogen bonding lowers energy. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From www.sciencephoto.com
Hydrogen fluoride molecule, illustration Stock Image F030/5813 Hydrogen Fluoride Is A Liquid At Room Temperature Due To As an anhydrous material, it is a colorless gas or fuming liquid. It is usually shipped in steel cylinders as a compressed. Hf is a weak acid, but its high solubility can be. Anhydrous hf has a boiling point of 19.5°c or 67°f. Because its boiling point is just. Hydrogen fluoride is a liquid, and so it can be described. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From www.alamy.com
Hydrofluoric acid, hydrofluoride, HF molecule. It is solution of Hydrogen Fluoride Is A Liquid At Room Temperature Due To Hf is a weak acid, but its high solubility can be. Hydrogen fluoride is a chemical compound that contains fluorine. Hydrogen fluoride is a colorless, fuming liquid or gas with a strong, irritating odor. Hydrogen fluoride is a liquid, and so it can be described as miscible with water. Such hydrogen bonding is absent in other. When mixed with water. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From www.rzepa.net
Autoionization of hydrogen fluoride. « Henry Rzepa's blog Hydrogen Fluoride Is A Liquid At Room Temperature Due To Hf is a weak acid, but its high solubility can be. Because its boiling point is just. Formation of hydrogen bonding lowers energy of the system and so hf is liquid. Hydrogen fluoride is a liquid, and so it can be described as miscible with water. When mixed with water it is called ‘hydrofluoric acid’. Hydrogen fluoride is a chemical. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From www.slideshare.net
Hydrofluoric acid Hydrogen Fluoride Is A Liquid At Room Temperature Due To When mixed with water it is called ‘hydrofluoric acid’. Hydrogen fluoride is a liquid, and so it can be described as miscible with water. Hf is a weak acid, but its high solubility can be. Hydrogen fluoride is a chemical compound that contains fluorine. Because its boiling point is just. It can exist as a colorless gas or as a. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From proper-cooking.info
Hydrogen Fluoride Lewis Structure Hydrogen Fluoride Is A Liquid At Room Temperature Due To Formation of hydrogen bonding lowers energy of the system and so hf is liquid. Pure hydrogen fluoride is a colourless, fuming liquid that boils at 19.5°c. Hf is a weak acid, but its high solubility can be. Hydrogen fluoride is a liquid, and so it can be described as miscible with water. Such hydrogen bonding is absent in other. Hydrogen. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From www.numerade.com
⏩SOLVEDAt room temperature, the molar volume of hydrogen fluoride Hydrogen Fluoride Is A Liquid At Room Temperature Due To Hydrogen fluoride is a chemical compound that contains fluorine. Formation of hydrogen bonding lowers energy of the system and so hf is liquid. Pure hydrogen fluoride is a colourless, fuming liquid that boils at 19.5°c. It can exist as a colorless gas or as a fuming liquid, or it can be. Hf is a weak acid, but its high solubility. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From byjus.com
At room temperature, the molar volume of hydrogen fluoride has a mass Hydrogen Fluoride Is A Liquid At Room Temperature Due To Hydrogen fluoride is a colorless, fuming liquid or gas with a strong, irritating odor. Hydrogen fluoride is a liquid, and so it can be described as miscible with water. Hydrogen fluoride is a chemical compound that contains fluorine. Formation of hydrogen bonding lowers energy of the system and so hf is liquid. As an anhydrous material, it is a colorless. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From www.vectorstock.com
HF hydrogen fluoride molecule Royalty Free Vector Image Hydrogen Fluoride Is A Liquid At Room Temperature Due To Because its boiling point is just. Due to strong and extensive hydrogen bonding, it boils at near room temperature, which is much higher of a temperature than other hydrogen. It can exist as a colorless gas or as a fuming liquid, or it can be. Pure hydrogen fluoride is a colourless, fuming liquid that boils at 19.5°c. Hf is a. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From techiescientist.com
Is HF (Hydrogen Fluoride) Ionic or Covalent? Techiescientist Hydrogen Fluoride Is A Liquid At Room Temperature Due To It can exist as a colorless gas or as a fuming liquid, or it can be. Hydrogen fluoride is a colorless, fuming liquid or gas with a strong, irritating odor. Hydrogen fluoride is a liquid, and so it can be described as miscible with water. Hydrogen fluoride is a chemical compound that contains fluorine. Formation of hydrogen bonding lowers energy. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From www.alamy.com
Hydrogen fluoride is a chemical compound with the formula HF. This Hydrogen Fluoride Is A Liquid At Room Temperature Due To When mixed with water it is called ‘hydrofluoric acid’. Hf is a weak acid, but its high solubility can be. Formation of hydrogen bonding lowers energy of the system and so hf is liquid. Because its boiling point is just. Hydrogen fluoride is a liquid, and so it can be described as miscible with water. Such hydrogen bonding is absent. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From www.sciencephoto.com
Hydrogen fluoride molecule Stock Image C045/7613 Science Photo Hydrogen Fluoride Is A Liquid At Room Temperature Due To It is usually shipped in steel cylinders as a compressed. Hydrogen fluoride is a chemical compound that contains fluorine. Due to strong and extensive hydrogen bonding, it boils at near room temperature, which is much higher of a temperature than other hydrogen. Pure hydrogen fluoride is a colourless, fuming liquid that boils at 19.5°c. It can exist as a colorless. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From www.shutterstock.com
Hydrogen Fluoride Hf Molecule Skeletal Formula Stock Vector (Royalty Hydrogen Fluoride Is A Liquid At Room Temperature Due To Anhydrous hf has a boiling point of 19.5°c or 67°f. Such hydrogen bonding is absent in other. When mixed with water it is called ‘hydrofluoric acid’. Hf is a weak acid, but its high solubility can be. It is usually shipped in steel cylinders as a compressed. Hydrogen fluoride is a chemical compound that contains fluorine. Pure hydrogen fluoride is. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From www.youtube.com
How to Write the Formula for Hydrogen fluoride YouTube Hydrogen Fluoride Is A Liquid At Room Temperature Due To Hf is a weak acid, but its high solubility can be. Formation of hydrogen bonding lowers energy of the system and so hf is liquid. Pure hydrogen fluoride is a colourless, fuming liquid that boils at 19.5°c. When mixed with water it is called ‘hydrofluoric acid’. Because its boiling point is just. Hydrogen fluoride is a liquid, and so it. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From www.boreal-laser.com
Hydrogen Fluoride Gas Detection Boreal Laser Hydrogen Fluoride Is A Liquid At Room Temperature Due To Pure hydrogen fluoride is a colourless, fuming liquid that boils at 19.5°c. Anhydrous hf has a boiling point of 19.5°c or 67°f. Hf is a weak acid, but its high solubility can be. It can exist as a colorless gas or as a fuming liquid, or it can be. Hydrogen fluoride is a chemical compound that contains fluorine. When mixed. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From www.numerade.com
SOLVED 15. The normal boiling points of ammonia, water, and hydrogen Hydrogen Fluoride Is A Liquid At Room Temperature Due To Because its boiling point is just. Pure hydrogen fluoride is a colourless, fuming liquid that boils at 19.5°c. Such hydrogen bonding is absent in other. Hf is a weak acid, but its high solubility can be. Hydrogen fluoride is a chemical compound that contains fluorine. Hydrogen fluoride is a liquid, and so it can be described as miscible with water.. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From www.pnas.org
Evidence of a liquidliquid phase transition in hot dense hydrogen PNAS Hydrogen Fluoride Is A Liquid At Room Temperature Due To Anhydrous hf has a boiling point of 19.5°c or 67°f. Hydrogen fluoride is a liquid, and so it can be described as miscible with water. As an anhydrous material, it is a colorless gas or fuming liquid. It can exist as a colorless gas or as a fuming liquid, or it can be. Hf is a weak acid, but its. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From dokumen.tips
(PDF) Room Temperature Hydrogen Storage in NanoConfined Liquids Hydrogen Fluoride Is A Liquid At Room Temperature Due To As an anhydrous material, it is a colorless gas or fuming liquid. When mixed with water it is called ‘hydrofluoric acid’. Hydrogen fluoride is a liquid, and so it can be described as miscible with water. It can exist as a colorless gas or as a fuming liquid, or it can be. Formation of hydrogen bonding lowers energy of the. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From www.pinterest.com
Happy MOLECULEMONDAY guys! Hydrogen fluoride is a chemical compound Hydrogen Fluoride Is A Liquid At Room Temperature Due To It can exist as a colorless gas or as a fuming liquid, or it can be. Hf is a weak acid, but its high solubility can be. Formation of hydrogen bonding lowers energy of the system and so hf is liquid. As an anhydrous material, it is a colorless gas or fuming liquid. Because its boiling point is just. Anhydrous. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From www.studypool.com
SOLUTION Chemistry in liquid hydrogen fluoride Studypool Hydrogen Fluoride Is A Liquid At Room Temperature Due To Pure hydrogen fluoride is a colourless, fuming liquid that boils at 19.5°c. As an anhydrous material, it is a colorless gas or fuming liquid. When mixed with water it is called ‘hydrofluoric acid’. Hydrogen fluoride is a liquid, and so it can be described as miscible with water. Hydrogen fluoride is a chemical compound that contains fluorine. Anhydrous hf has. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From utedzz.blogspot.com
Periodic Table Liquids At Room Temp Periodic Table Timeline Hydrogen Fluoride Is A Liquid At Room Temperature Due To Such hydrogen bonding is absent in other. Because its boiling point is just. Formation of hydrogen bonding lowers energy of the system and so hf is liquid. Hf is a weak acid, but its high solubility can be. Hydrogen fluoride is a liquid, and so it can be described as miscible with water. As an anhydrous material, it is a. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From www.youtube.com
Draw the Lewis Structure of HF (hydrogen fluoride) YouTube Hydrogen Fluoride Is A Liquid At Room Temperature Due To Such hydrogen bonding is absent in other. When mixed with water it is called ‘hydrofluoric acid’. Hf is a weak acid, but its high solubility can be. Pure hydrogen fluoride is a colourless, fuming liquid that boils at 19.5°c. As an anhydrous material, it is a colorless gas or fuming liquid. Anhydrous hf has a boiling point of 19.5°c or. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From pixels.com
Hydrogen Fluoride Molecule Photograph by Molekuul/science Photo Library Hydrogen Fluoride Is A Liquid At Room Temperature Due To It is usually shipped in steel cylinders as a compressed. Hf is a weak acid, but its high solubility can be. Anhydrous hf has a boiling point of 19.5°c or 67°f. It can exist as a colorless gas or as a fuming liquid, or it can be. Such hydrogen bonding is absent in other. Hydrogen fluoride is a chemical compound. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From www.wbir.com
What is hydrogen fluoride? Hydrogen Fluoride Is A Liquid At Room Temperature Due To Because its boiling point is just. Hydrogen fluoride is a chemical compound that contains fluorine. It is usually shipped in steel cylinders as a compressed. As an anhydrous material, it is a colorless gas or fuming liquid. Due to strong and extensive hydrogen bonding, it boils at near room temperature, which is much higher of a temperature than other hydrogen.. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From mungfali.com
Hydrogen Fluoride Lewis Structure Hydrogen Fluoride Is A Liquid At Room Temperature Due To When mixed with water it is called ‘hydrofluoric acid’. It is usually shipped in steel cylinders as a compressed. As an anhydrous material, it is a colorless gas or fuming liquid. Hf is a weak acid, but its high solubility can be. Such hydrogen bonding is absent in other. Hydrogen fluoride is a colorless, fuming liquid or gas with a. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From www.researchgate.net
Phase diagram of hydrogen. The pressure path at room temperature in the Hydrogen Fluoride Is A Liquid At Room Temperature Due To Pure hydrogen fluoride is a colourless, fuming liquid that boils at 19.5°c. Hydrogen fluoride is a chemical compound that contains fluorine. Such hydrogen bonding is absent in other. It can exist as a colorless gas or as a fuming liquid, or it can be. As an anhydrous material, it is a colorless gas or fuming liquid. It is usually shipped. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From www.numerade.com
SOLVEDHydrogen fluoride is a liquid unlike other hydrogen halides Hydrogen Fluoride Is A Liquid At Room Temperature Due To Hf is a weak acid, but its high solubility can be. Formation of hydrogen bonding lowers energy of the system and so hf is liquid. As an anhydrous material, it is a colorless gas or fuming liquid. Such hydrogen bonding is absent in other. Hydrogen fluoride is a liquid, and so it can be described as miscible with water. Hydrogen. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From www.bigstockphoto.com
Hydrogen Fluoride Image & Photo (Free Trial) Bigstock Hydrogen Fluoride Is A Liquid At Room Temperature Due To Because its boiling point is just. Due to strong and extensive hydrogen bonding, it boils at near room temperature, which is much higher of a temperature than other hydrogen. Hf is a weak acid, but its high solubility can be. Such hydrogen bonding is absent in other. Hydrogen fluoride is a liquid, and so it can be described as miscible. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From guides.hostos.cuny.edu
Chapter 3 Solids and Liquids CHE 110 Introduction to Chemistry Hydrogen Fluoride Is A Liquid At Room Temperature Due To Hf is a weak acid, but its high solubility can be. As an anhydrous material, it is a colorless gas or fuming liquid. It can exist as a colorless gas or as a fuming liquid, or it can be. Formation of hydrogen bonding lowers energy of the system and so hf is liquid. Due to strong and extensive hydrogen bonding,. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From edu.rsc.org
Chemical misconceptions II Hydrogen fluoride Resource RSC Education Hydrogen Fluoride Is A Liquid At Room Temperature Due To It is usually shipped in steel cylinders as a compressed. Because its boiling point is just. Hf is a weak acid, but its high solubility can be. Hydrogen fluoride is a liquid, and so it can be described as miscible with water. Due to strong and extensive hydrogen bonding, it boils at near room temperature, which is much higher of. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From askfilo.com
(29. Hydrogen fluoride is a liquid at room temperature dueto Filo Hydrogen Fluoride Is A Liquid At Room Temperature Due To Due to strong and extensive hydrogen bonding, it boils at near room temperature, which is much higher of a temperature than other hydrogen. Formation of hydrogen bonding lowers energy of the system and so hf is liquid. When mixed with water it is called ‘hydrofluoric acid’. Because its boiling point is just. It is usually shipped in steel cylinders as. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.
From matmake.com
Hydrogen Fluoride Properties Hydrogen Fluoride Is A Liquid At Room Temperature Due To Hf is a weak acid, but its high solubility can be. Hydrogen fluoride is a colorless, fuming liquid or gas with a strong, irritating odor. Hydrogen fluoride is a liquid, and so it can be described as miscible with water. It is usually shipped in steel cylinders as a compressed. It can exist as a colorless gas or as a. Hydrogen Fluoride Is A Liquid At Room Temperature Due To.