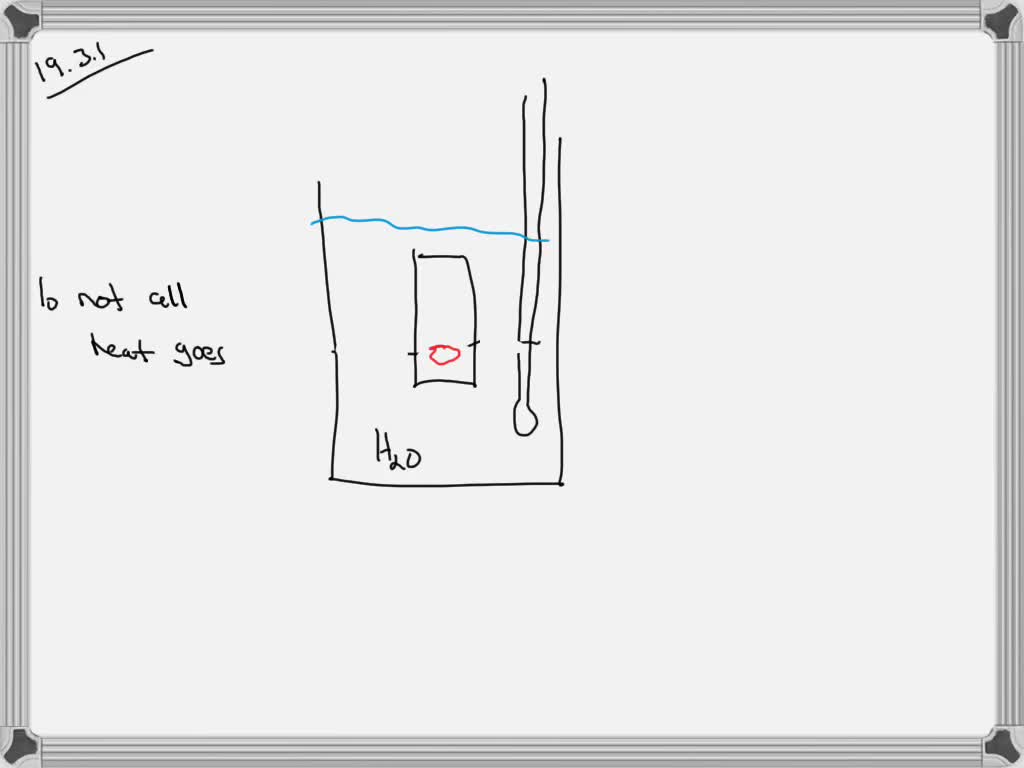
Calorimeter Lab Sources Of Error . Learn how to measure the enthalpy changes of two reactions using constant pressure calorimetry and apply hess's law to calculate the enthalpy of combustion of a. Explore the concepts, equations, and examples of calorimetry, and the sources of. To do so, the heat is exchanged with a calibrated object. In this lab, you will do two classic calorimetry experiments: Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. (an average deviation of 0.02 j/g°c and a relative. Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. Calorimetry is used to measure amounts of heat transferred to or from a substance. Learn how to use calorimeters to measure the amount of heat involved in chemical or physical processes. Use your calculated average deviation and relative error in your discussion.
from www.numerade.com
Learn how to use calorimeters to measure the amount of heat involved in chemical or physical processes. (an average deviation of 0.02 j/g°c and a relative. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. Explore the concepts, equations, and examples of calorimetry, and the sources of. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. In this lab, you will do two classic calorimetry experiments: Learn how to measure the enthalpy changes of two reactions using constant pressure calorimetry and apply hess's law to calculate the enthalpy of combustion of a. Use your calculated average deviation and relative error in your discussion. Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference.
Name three possible sources of error in a calorimetry experiment
Calorimeter Lab Sources Of Error (an average deviation of 0.02 j/g°c and a relative. (an average deviation of 0.02 j/g°c and a relative. Calorimetry is used to measure amounts of heat transferred to or from a substance. Use your calculated average deviation and relative error in your discussion. Learn how to measure the enthalpy changes of two reactions using constant pressure calorimetry and apply hess's law to calculate the enthalpy of combustion of a. Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. Learn how to use calorimeters to measure the amount of heat involved in chemical or physical processes. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. Explore the concepts, equations, and examples of calorimetry, and the sources of. To do so, the heat is exchanged with a calibrated object. In this lab, you will do two classic calorimetry experiments:
From www.scribd.com
Determining Specific Heat Capacity Through Calorimetry An Analysis of Calorimeter Lab Sources Of Error Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. Learn how to measure the enthalpy changes of two reactions using constant pressure calorimetry and apply hess's law to calculate the enthalpy of combustion of a. To do so, the heat is exchanged with a calibrated object. In this lab, you will do. Calorimeter Lab Sources Of Error.
From www.savemyexams.com
Calorimetry Experiments SL IB Chemistry Revision Notes 2025 Save My Calorimeter Lab Sources Of Error Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. In this lab, you will do two classic calorimetry experiments: Learn how to use calorimeters to measure the amount of heat involved in chemical or physical processes. Calorimetry is used to measure amounts of heat transferred to or from a substance. Measuring the. Calorimeter Lab Sources Of Error.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Calorimeter Lab Sources Of Error In this lab, you will do two classic calorimetry experiments: Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. Learn how to measure the enthalpy changes of two reactions using constant pressure calorimetry and apply hess's law to calculate the enthalpy of combustion of a. Explore the concepts, equations, and examples of. Calorimeter Lab Sources Of Error.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimeter Lab Sources Of Error Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. Use your calculated average deviation and relative error in your discussion. Learn how to measure the enthalpy changes of two reactions using constant pressure calorimetry and apply hess's law to calculate the enthalpy of combustion of a. Explore the concepts, equations, and. Calorimeter Lab Sources Of Error.
From www.numerade.com
Name three possible sources of error in a calorimetry experiment Calorimeter Lab Sources Of Error Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. Use your calculated average deviation and relative error in your discussion. In this lab, you will do two classic calorimetry experiments: Explore the concepts, equations,. Calorimeter Lab Sources Of Error.
From www.nagwa.com
Question Video Identifying the Factor That Does Not Need to Be Held Calorimeter Lab Sources Of Error In this lab, you will do two classic calorimetry experiments: Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. Learn how to use calorimeters to measure the amount of heat involved in chemical or physical processes. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two. Calorimeter Lab Sources Of Error.
From www.studypool.com
SOLUTION Calorimetry Enthalpy of Reaction and Heat Capacity of a Calorimeter Lab Sources Of Error Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. Learn how to use calorimeters to measure the amount of heat involved in chemical or physical processes. To do so, the heat is exchanged with a calibrated object. Learn how to measure the enthalpy changes of two reactions using constant pressure calorimetry. Calorimeter Lab Sources Of Error.
From studylib.net
Calorimetry Experiment and Practice Problems for Virtual Lab Key Calorimeter Lab Sources Of Error Calorimetry is used to measure amounts of heat transferred to or from a substance. (an average deviation of 0.02 j/g°c and a relative. Explore the concepts, equations, and examples of calorimetry, and the sources of. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. Learn how to use calorimeters to measure. Calorimeter Lab Sources Of Error.
From www.studypool.com
SOLUTION Calorimetry Enthalpy of Reaction and Heat Capacity of a Calorimeter Lab Sources Of Error In this lab, you will do two classic calorimetry experiments: Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. Explore the concepts, equations, and examples of calorimetry, and the sources of. Calorimetry is used to measure amounts of heat transferred to or from a substance. Learn how to use calorimeters to. Calorimeter Lab Sources Of Error.
From ecampusontario.pressbooks.pub
5.2 Calorimetry Chemistry Calorimeter Lab Sources Of Error To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. Explore the concepts, equations, and examples of calorimetry, and the sources of. In this lab, you will do two classic calorimetry experiments: Use your calculated average deviation and relative error in your discussion. Learn how. Calorimeter Lab Sources Of Error.
From www.scribd.com
EXPERIMENT 302 Heat and Calorimetry Analysis Sources of Error PDF Calorimeter Lab Sources Of Error Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. Use your calculated average deviation and relative error in your discussion. (an average deviation of 0.02 j/g°c and a relative. Learn how to measure the enthalpy changes of two reactions using constant pressure calorimetry and apply hess's law to calculate the enthalpy of. Calorimeter Lab Sources Of Error.
From www.slideshare.net
4 Calorimetry Calorimeter Lab Sources Of Error Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. Calorimetry is used to measure amounts of heat transferred to or from a substance. Explore the concepts, equations, and examples of calorimetry, and the sources of. Learn how to measure the enthalpy changes of two reactions using constant pressure calorimetry and apply hess's. Calorimeter Lab Sources Of Error.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Calorimeter Lab Sources Of Error To do so, the heat is exchanged with a calibrated object. Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. Learn how to use calorimeters to measure the amount of heat involved in chemical or physical processes. Explore the concepts, equations, and examples of calorimetry, and the sources of. Calorimetry is used. Calorimeter Lab Sources Of Error.
From www.pdfprof.com
determination of calorimeter constant lab report Calorimeter Lab Sources Of Error In this lab, you will do two classic calorimetry experiments: (an average deviation of 0.02 j/g°c and a relative. Learn how to measure the enthalpy changes of two reactions using constant pressure calorimetry and apply hess's law to calculate the enthalpy of combustion of a. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of. Calorimeter Lab Sources Of Error.
From www.researchgate.net
Sources of error in calorimetry measurements Download Scientific Diagram Calorimeter Lab Sources Of Error In this lab, you will do two classic calorimetry experiments: Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. To do so, the heat is exchanged with a calibrated object. Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. Learn how to measure. Calorimeter Lab Sources Of Error.
From chemwiki.ucdavis.edu
Chapter 9.6 Calorimetry Chemwiki Calorimeter Lab Sources Of Error Learn how to measure the enthalpy changes of two reactions using constant pressure calorimetry and apply hess's law to calculate the enthalpy of combustion of a. Calorimetry is used to measure amounts of heat transferred to or from a substance. Explore the concepts, equations, and examples of calorimetry, and the sources of. (an average deviation of 0.02 j/g°c and a. Calorimeter Lab Sources Of Error.
From www.studocu.com
Calorimetry LAB Question 1 1. In part I of the experiment, what is a Calorimeter Lab Sources Of Error Learn how to use calorimeters to measure the amount of heat involved in chemical or physical processes. Learn how to measure the enthalpy changes of two reactions using constant pressure calorimetry and apply hess's law to calculate the enthalpy of combustion of a. Explore the concepts, equations, and examples of calorimetry, and the sources of. (an average deviation of 0.02. Calorimeter Lab Sources Of Error.
From www.slideserve.com
PPT Chapter 14 Heat PowerPoint Presentation, free download ID6832402 Calorimeter Lab Sources Of Error To do so, the heat is exchanged with a calibrated object. In this lab, you will do two classic calorimetry experiments: Explore the concepts, equations, and examples of calorimetry, and the sources of. Learn how to measure the enthalpy changes of two reactions using constant pressure calorimetry and apply hess's law to calculate the enthalpy of combustion of a. (an. Calorimeter Lab Sources Of Error.
From www.numerade.com
SOLVED Name three possible sources of error in a calorimetry experiment. Calorimeter Lab Sources Of Error Learn how to measure the enthalpy changes of two reactions using constant pressure calorimetry and apply hess's law to calculate the enthalpy of combustion of a. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. Learn how to use calorimeters to measure the amount of heat involved in chemical or physical. Calorimeter Lab Sources Of Error.
From studylib.net
5.1 b) CALORIMETRY Calorimeter Lab Sources Of Error Use your calculated average deviation and relative error in your discussion. Learn how to measure the enthalpy changes of two reactions using constant pressure calorimetry and apply hess's law to calculate the enthalpy of combustion of a. (an average deviation of 0.02 j/g°c and a relative. Calorimetry is used to measure amounts of heat transferred to or from a substance.. Calorimeter Lab Sources Of Error.
From www.slideserve.com
PPT Chapter 12 “Heat in Chemical Reactions” PowerPoint Presentation Calorimeter Lab Sources Of Error To do so, the heat is exchanged with a calibrated object. Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. (an average deviation of 0.02 j/g°c and a relative. In this lab, you will do two classic calorimetry experiments: Learn how to measure the enthalpy changes of two reactions using constant pressure. Calorimeter Lab Sources Of Error.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimeter Lab Sources Of Error Learn how to use calorimeters to measure the amount of heat involved in chemical or physical processes. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. In this lab, you will do two classic calorimetry experiments: Explore the concepts, equations, and examples of calorimetry, and the sources of. Learn how to. Calorimeter Lab Sources Of Error.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Calorimeter Lab Sources Of Error In this lab, you will do two classic calorimetry experiments: Explore the concepts, equations, and examples of calorimetry, and the sources of. Calorimetry is used to measure amounts of heat transferred to or from a substance. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. Use your calculated average deviation and. Calorimeter Lab Sources Of Error.
From www.youtube.com
R1.1.4 What are the sources of error in calorimetry? YouTube Calorimeter Lab Sources Of Error Learn how to measure the enthalpy changes of two reactions using constant pressure calorimetry and apply hess's law to calculate the enthalpy of combustion of a. Learn how to use calorimeters to measure the amount of heat involved in chemical or physical processes. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different. Calorimeter Lab Sources Of Error.
From www.slideshare.net
4 Calorimetry Calorimeter Lab Sources Of Error Explore the concepts, equations, and examples of calorimetry, and the sources of. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. Learn how to use calorimeters. Calorimeter Lab Sources Of Error.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimeter Lab Sources Of Error Calorimetry is used to measure amounts of heat transferred to or from a substance. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. Learn how to use calorimeters to measure the amount of heat involved in chemical or physical processes. Use your calculated average deviation and relative error in your discussion.. Calorimeter Lab Sources Of Error.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Lab Sources Of Error In this lab, you will do two classic calorimetry experiments: Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. Use your calculated average deviation and relative error in your discussion. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. Explore the concepts, equations,. Calorimeter Lab Sources Of Error.
From studylib.net
Lab Calorimetry High School Science Help Calorimeter Lab Sources Of Error (an average deviation of 0.02 j/g°c and a relative. Learn how to measure the enthalpy changes of two reactions using constant pressure calorimetry and apply hess's law to calculate the enthalpy of combustion of a. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance.. Calorimeter Lab Sources Of Error.
From tukioka-clinic.com
😂 Soda can calorimeter sources of error. What Is a Calorimeter & What Calorimeter Lab Sources Of Error Calorimetry is used to measure amounts of heat transferred to or from a substance. Use your calculated average deviation and relative error in your discussion. Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. (an average deviation of 0.02 j/g°c and a relative. Learn how to measure the enthalpy changes of two. Calorimeter Lab Sources Of Error.
From www.studocu.com
Calorimetry Lab SE Lab General Physics 1 Studocu Calorimeter Lab Sources Of Error In this lab, you will do two classic calorimetry experiments: Calorimetry is used to measure amounts of heat transferred to or from a substance. Learn how to use calorimeters to measure the amount of heat involved in chemical or physical processes. Use your calculated average deviation and relative error in your discussion. To do so, the heat is exchanged with. Calorimeter Lab Sources Of Error.
From www.youtube.com
Effects of experimental errors on enthalpy measurements YouTube Calorimeter Lab Sources Of Error To do so, the heat is exchanged with a calibrated object. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. In this lab, you will do two classic calorimetry experiments: Explore the concepts, equations,. Calorimeter Lab Sources Of Error.
From www.education.com
Calorimetry Bomb Calorimeter Experiment Calorimeter Lab Sources Of Error Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. Calorimetry is used to measure amounts of heat transferred to or from a substance. Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. To do so, the heat is exchanged with a calibrated object.. Calorimeter Lab Sources Of Error.
From www.scribd.com
Calorimetry Lab Heat Capacity Heat Calorimeter Lab Sources Of Error Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. (an average deviation of 0.02 j/g°c and a relative. Explore the concepts, equations, and examples of calorimetry, and the sources of. Learn how to measure the enthalpy changes of two reactions using constant pressure calorimetry and apply hess's law to calculate the enthalpy. Calorimeter Lab Sources Of Error.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Calorimeter Lab Sources Of Error Learn how to measure the enthalpy changes of two reactions using constant pressure calorimetry and apply hess's law to calculate the enthalpy of combustion of a. Learn how to use calorimeters to measure the amount of heat involved in chemical or physical processes. Use your calculated average deviation and relative error in your discussion. (an average deviation of 0.02 j/g°c. Calorimeter Lab Sources Of Error.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimeter Lab Sources Of Error Learn how to measure and calculate error in science experiments using absolute, relative, percent and percent difference. Explore the concepts, equations, and examples of calorimetry, and the sources of. (an average deviation of 0.02 j/g°c and a relative. In this lab, you will do two classic calorimetry experiments: Learn how to use calorimeters to measure the amount of heat involved. Calorimeter Lab Sources Of Error.