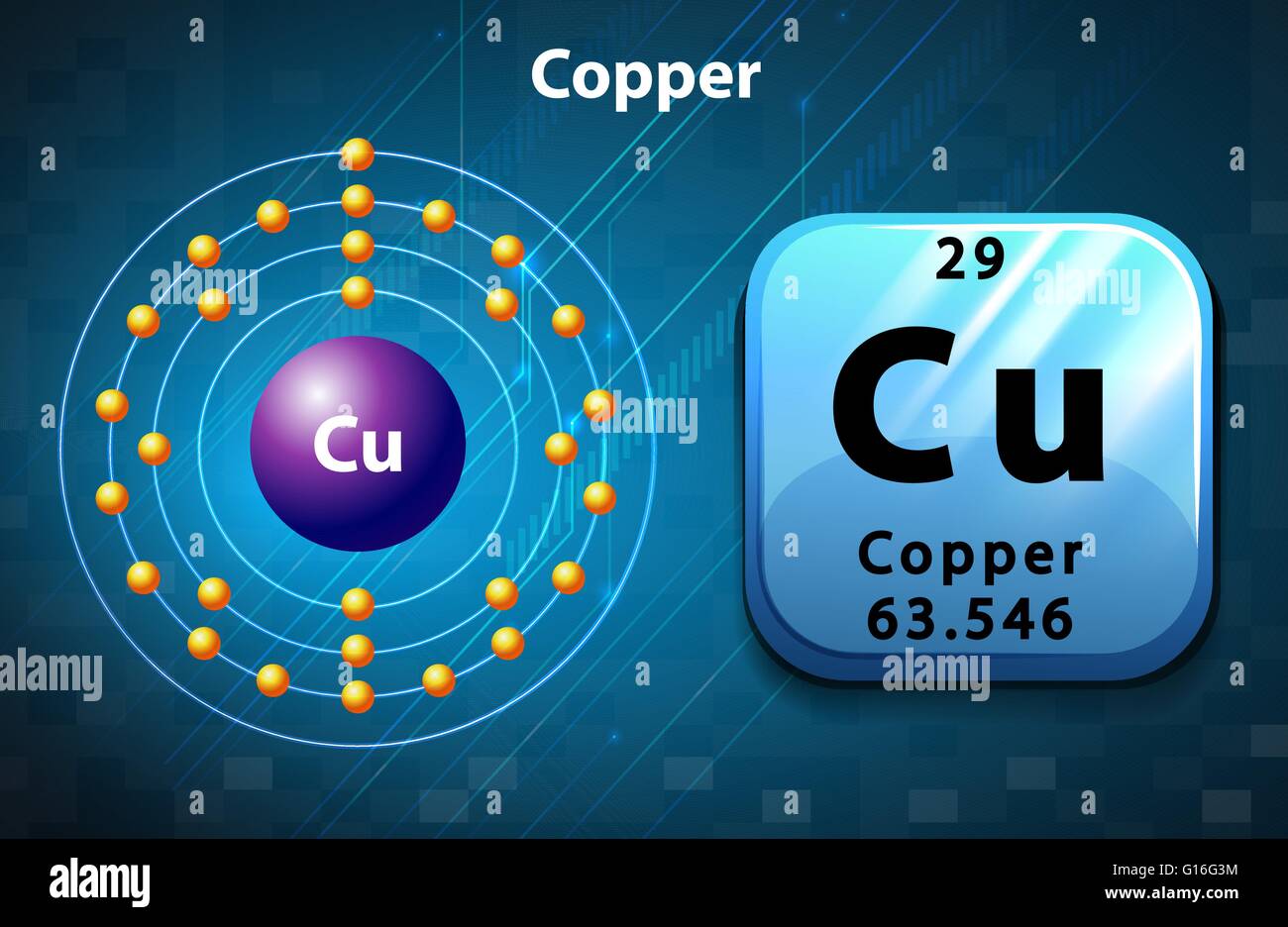
Copper Metal Free Electrons . metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. Clearly, the “free” electrons in a metal are. copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. the number of free electrons in a metal is of the same order as the number of atoms: The precise number, however, depends on the. describe the classical free electron model of metals in terms of the concept electron number density. Electrons do not pop out of a piece of copper lying on the table.
from www.alamy.com
The precise number, however, depends on the. metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. Electrons do not pop out of a piece of copper lying on the table. describe the classical free electron model of metals in terms of the concept electron number density. the number of free electrons in a metal is of the same order as the number of atoms: Clearly, the “free” electrons in a metal are. copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules.
Copper atom hires stock photography and images Alamy
Copper Metal Free Electrons Electrons do not pop out of a piece of copper lying on the table. The precise number, however, depends on the. describe the classical free electron model of metals in terms of the concept electron number density. the number of free electrons in a metal is of the same order as the number of atoms: copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. Electrons do not pop out of a piece of copper lying on the table. metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. Clearly, the “free” electrons in a metal are.
From elchoroukhost.net
Copper Periodic Table Protons Neutrons And Electrons Elcho Table Copper Metal Free Electrons copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. the number of free electrons in a metal is of the same order as the number of atoms: metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. Electrons do not pop out. Copper Metal Free Electrons.
From dxojzvsyh.blob.core.windows.net
Copper Electron Cloud Model at Wallace Miles blog Copper Metal Free Electrons the number of free electrons in a metal is of the same order as the number of atoms: Electrons do not pop out of a piece of copper lying on the table. describe the classical free electron model of metals in terms of the concept electron number density. copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard. Copper Metal Free Electrons.
From www.youtube.com
Electrical Conductivity of Metals Free electron theory III B.Sc. PMCs Class I YouTube Copper Metal Free Electrons Clearly, the “free” electrons in a metal are. Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. Electrons do not pop out of a piece of copper lying on the table. describe the classical free electron model of metals in terms of the concept electron number density. The precise. Copper Metal Free Electrons.
From www.youtube.com
Drude Lorentz / Electron Pool Theory of Metals Free Electron YouTube Copper Metal Free Electrons Electrons do not pop out of a piece of copper lying on the table. metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. Clearly, the “free” electrons in a metal are. Metals, such as copper and aluminum, are held together by bonds that are very different from those of. Copper Metal Free Electrons.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Copper Metal Free Electrons copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. Electrons do not pop out of a piece of copper lying on the table. describe the classical free electron model of metals in terms of the concept electron number density. The precise number, however, depends on the. Metals, such as copper and aluminum,. Copper Metal Free Electrons.
From www.youtube.com
Copper Electron Configuration Organic Chemistry Examples YouTube Copper Metal Free Electrons copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. the number of free electrons in a metal is of the same order as the number of atoms: Electrons do not pop out of a piece of copper lying on the table. metals, such as copper and aluminum, are held together by. Copper Metal Free Electrons.
From www.researchgate.net
Electronic properties of metals in increasing order of free electron... Download Scientific Copper Metal Free Electrons Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. describe the classical free. Copper Metal Free Electrons.
From www.toppr.com
Assume that each atom of copper contributes one free electron. The density of copper is 9g cm^3 Copper Metal Free Electrons describe the classical free electron model of metals in terms of the concept electron number density. the number of free electrons in a metal is of the same order as the number of atoms: Clearly, the “free” electrons in a metal are. Metals, such as copper and aluminum, are held together by bonds that are very different from. Copper Metal Free Electrons.
From www.youtube.com
How to Find the Valence Electrons for Copper (Cu) YouTube Copper Metal Free Electrons Electrons do not pop out of a piece of copper lying on the table. The precise number, however, depends on the. metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. the number. Copper Metal Free Electrons.
From mammothmemory.net
Metal atoms are held strongly where electrons move freely Copper Metal Free Electrons metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. Electrons do not pop out of a piece of copper lying on the table. Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. Clearly, the “free” electrons in a metal. Copper Metal Free Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Copper Metal Free Electrons metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. Electrons do not pop out of a piece of copper lying on the table. Clearly, the “free” electrons in a metal are. describe. Copper Metal Free Electrons.
From www.slideserve.com
PPT Metals I Free Electron Model PowerPoint Presentation, free download ID3222746 Copper Metal Free Electrons the number of free electrons in a metal is of the same order as the number of atoms: Clearly, the “free” electrons in a metal are. The precise number, however, depends on the. Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating. Copper Metal Free Electrons.
From www.studypool.com
SOLUTION Free electron theory of metals Studypool Copper Metal Free Electrons The precise number, however, depends on the. metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. Electrons do not pop out of a piece of copper lying on the table. copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. the number. Copper Metal Free Electrons.
From www.slideserve.com
PPT ELECTRON THEORY OF METALS PowerPoint Presentation, free download ID3687751 Copper Metal Free Electrons Electrons do not pop out of a piece of copper lying on the table. describe the classical free electron model of metals in terms of the concept electron number density. The precise number, however, depends on the. Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. the number. Copper Metal Free Electrons.
From owlcation.com
Primary and Secondary Bonds Owlcation Copper Metal Free Electrons Clearly, the “free” electrons in a metal are. The precise number, however, depends on the. copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. Metals, such as copper and aluminum, are held together. Copper Metal Free Electrons.
From www.studypool.com
SOLUTION SU Classical Free Electron Theory Of Metals Presentation Studypool Copper Metal Free Electrons copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. describe the classical free electron model of metals in terms of the concept electron number density. The precise number, however, depends on the. Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. . Copper Metal Free Electrons.
From www.studocu.com
Chapter 4 Free electron theory of Metals Free electron theory of Metals Drude Lorentz model Copper Metal Free Electrons the number of free electrons in a metal is of the same order as the number of atoms: copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. The precise number, however, depends on the. metals, such as copper and aluminum, are held together by bonds that are very different from those. Copper Metal Free Electrons.
From www.studypool.com
SOLUTION Free electron theory of metals Studypool Copper Metal Free Electrons the number of free electrons in a metal is of the same order as the number of atoms: metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. Electrons do not pop out of a piece of copper lying on the table. describe the classical free electron model. Copper Metal Free Electrons.
From periodictable.me
How To Find A Electron Configuration For Copper Dynamic Periodic Table of Elements and Chemistry Copper Metal Free Electrons Clearly, the “free” electrons in a metal are. Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. the number of free electrons in a metal is of the same order as the number of atoms: The precise number, however, depends on the. describe the classical free electron model. Copper Metal Free Electrons.
From www.researchgate.net
Schematic of a Drude metal. Free electrons accelerate and scatter off... Download Scientific Copper Metal Free Electrons copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. describe the classical free electron model of metals in terms of the concept electron number density. Electrons do not pop out of a. Copper Metal Free Electrons.
From www.youtube.com
Electron gas in metals Free electron theory of metals and Fermi theory YouTube Copper Metal Free Electrons the number of free electrons in a metal is of the same order as the number of atoms: metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. Clearly, the. Copper Metal Free Electrons.
From www.slideserve.com
PPT Metals Free Electron Model PowerPoint Presentation, free download ID1071091 Copper Metal Free Electrons Clearly, the “free” electrons in a metal are. Electrons do not pop out of a piece of copper lying on the table. the number of free electrons in a metal is of the same order as the number of atoms: metals, such as copper and aluminum, are held together by bonds that are very different from those of. Copper Metal Free Electrons.
From www.studypool.com
SOLUTION Free electron theory of metals Studypool Copper Metal Free Electrons the number of free electrons in a metal is of the same order as the number of atoms: The precise number, however, depends on the. copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. Metals, such as copper and aluminum, are held together by bonds that are very different from those of. Copper Metal Free Electrons.
From www.sciencephoto.com
Copper, atomic structure Stock Image C018/3710 Science Photo Library Copper Metal Free Electrons the number of free electrons in a metal is of the same order as the number of atoms: Electrons do not pop out of a piece of copper lying on the table. Clearly, the “free” electrons in a metal are. copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. The precise number,. Copper Metal Free Electrons.
From www.studypool.com
SOLUTION Free electron theory of metals Studypool Copper Metal Free Electrons metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. describe the classical free electron model of metals in terms of the concept electron number density. the number of free electrons in. Copper Metal Free Electrons.
From www.studypool.com
SOLUTION Free electron theory of metals Studypool Copper Metal Free Electrons copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. the number of free electrons in a metal is of the same order as the number of atoms: Clearly, the “free” electrons in a metal are. metals, such as copper and aluminum, are held together by bonds that are very different from. Copper Metal Free Electrons.
From www.alamy.com
Copper atom hires stock photography and images Alamy Copper Metal Free Electrons Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. Electrons do not pop out of a piece of copper lying on the table. describe the classical free electron model of metals in terms of the concept electron number density. Clearly, the “free” electrons in a metal are. the. Copper Metal Free Electrons.
From www.mooramo.com
Ions of Transition Elements Mooramo Copper Metal Free Electrons copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. Clearly, the “free” electrons in a metal are. metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. describe the classical free electron model of metals in terms of the concept electron number. Copper Metal Free Electrons.
From www.slideserve.com
PPT Chapter 7 “Metallic Bonding” PowerPoint Presentation, free download ID1445720 Copper Metal Free Electrons Electrons do not pop out of a piece of copper lying on the table. The precise number, however, depends on the. Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. Clearly, the “free” electrons in a metal are. copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. Copper Metal Free Electrons.
From www.studypool.com
SOLUTION Free electron theory of metals Studypool Copper Metal Free Electrons Clearly, the “free” electrons in a metal are. copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. Electrons do not pop out of a piece of copper lying on the table. metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. Metals, such. Copper Metal Free Electrons.
From www.slideshare.net
Chapter 24 Conduction Copper Metal Free Electrons describe the classical free electron model of metals in terms of the concept electron number density. The precise number, however, depends on the. copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules.. Copper Metal Free Electrons.
From www.researchgate.net
The 'free electron' model of metals assumes that the conduction... Download Scientific Diagram Copper Metal Free Electrons Metals, such as copper and aluminum, are held together by bonds that are very different from those of molecules. Electrons do not pop out of a piece of copper lying on the table. describe the classical free electron model of metals in terms of the concept electron number density. metals, such as copper and aluminum, are held together. Copper Metal Free Electrons.
From www.slideserve.com
PPT Metals Free Electron Model PowerPoint Presentation, free download ID1071091 Copper Metal Free Electrons The precise number, however, depends on the. Clearly, the “free” electrons in a metal are. copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. describe the classical free electron model of metals in terms of the concept electron number density. metals, such as copper and aluminum, are held together by bonds. Copper Metal Free Electrons.
From www.youtube.com
Free electron theory of metals YouTube Copper Metal Free Electrons the number of free electrons in a metal is of the same order as the number of atoms: describe the classical free electron model of metals in terms of the concept electron number density. Electrons do not pop out of a piece of copper lying on the table. The precise number, however, depends on the. Clearly, the “free”. Copper Metal Free Electrons.
From www.studypool.com
SOLUTION Conduction in metals free electron model Studypool Copper Metal Free Electrons describe the classical free electron model of metals in terms of the concept electron number density. Clearly, the “free” electrons in a metal are. Electrons do not pop out of a piece of copper lying on the table. The precise number, however, depends on the. copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due. Copper Metal Free Electrons.