Why Are Ionic Compounds Soluble In Water Gcse . When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. See the study guide on the three. Polar solvent h 2 o dissociates the ionic compound by interacting with the charged ions and pulls them with a strong force of attraction. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. However, they often dissolve in water to form an aqueous solution. Ionic compounds have high melting and boiling points due to the strong forces of attraction between the ions. Ionic compounds are good conductors of electricity in the molten state or in solution when the ionic compound is melted or dissolved in. Close aqueous solution a solution in which the. At room temperature, ionic compounds are typically in a solid state. Most ionic compounds are soluble in water and form aqueous solutions.
from www.chegg.com
Most ionic compounds are soluble in water and form aqueous solutions. At room temperature, ionic compounds are typically in a solid state. Ionic compounds have high melting and boiling points due to the strong forces of attraction between the ions. Polar solvent h 2 o dissociates the ionic compound by interacting with the charged ions and pulls them with a strong force of attraction. Ionic compounds are good conductors of electricity in the molten state or in solution when the ionic compound is melted or dissolved in. See the study guide on the three. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. However, they often dissolve in water to form an aqueous solution. Close aqueous solution a solution in which the. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because.
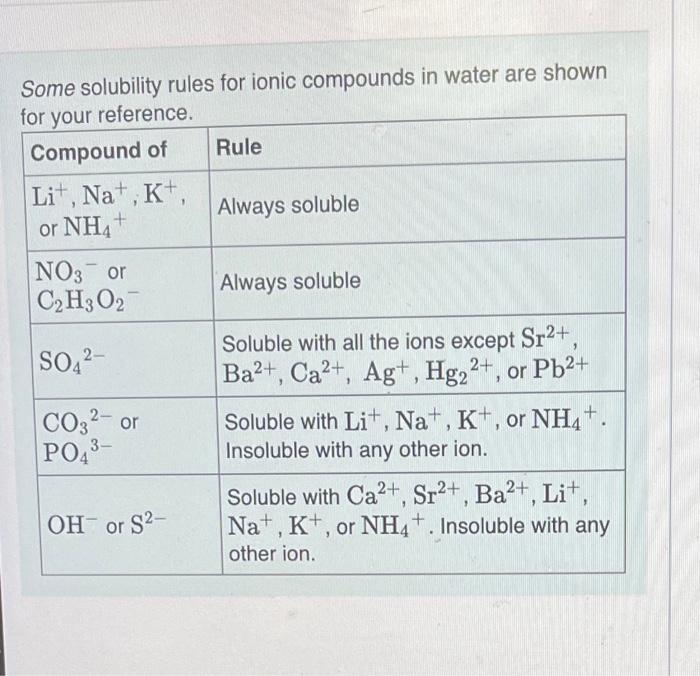
Solved Some solubility rules for ionic compounds in water
Why Are Ionic Compounds Soluble In Water Gcse Most ionic compounds are soluble in water and form aqueous solutions. Close aqueous solution a solution in which the. Polar solvent h 2 o dissociates the ionic compound by interacting with the charged ions and pulls them with a strong force of attraction. See the study guide on the three. Ionic compounds have high melting and boiling points due to the strong forces of attraction between the ions. At room temperature, ionic compounds are typically in a solid state. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. Most ionic compounds are soluble in water and form aqueous solutions. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. However, they often dissolve in water to form an aqueous solution. Ionic compounds are good conductors of electricity in the molten state or in solution when the ionic compound is melted or dissolved in.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download Why Are Ionic Compounds Soluble In Water Gcse Close aqueous solution a solution in which the. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. However, they often dissolve in water to form an aqueous solution. See the study guide on the three. At room temperature, ionic compounds are typically in a solid state. Ionic compounds have high. Why Are Ionic Compounds Soluble In Water Gcse.
From www.slideserve.com
PPT Solubility of Ionic Compounds PowerPoint Presentation, free Why Are Ionic Compounds Soluble In Water Gcse When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. Polar solvent h 2 o dissociates the ionic compound by interacting with the charged ions and pulls them with a strong force of attraction. Ionic compounds are good conductors of electricity in the molten state or in solution when the ionic. Why Are Ionic Compounds Soluble In Water Gcse.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download Why Are Ionic Compounds Soluble In Water Gcse At room temperature, ionic compounds are typically in a solid state. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. However, they often dissolve in water to form an aqueous solution. Most ionic compounds are soluble in water and form aqueous solutions. Polar solvent h 2 o dissociates the ionic. Why Are Ionic Compounds Soluble In Water Gcse.
From www.youtube.com
Why are ionic compounds soluble in water? YouTube Why Are Ionic Compounds Soluble In Water Gcse Polar solvent h 2 o dissociates the ionic compound by interacting with the charged ions and pulls them with a strong force of attraction. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Ionic compounds are good conductors of electricity in the molten state or in solution when the ionic compound. Why Are Ionic Compounds Soluble In Water Gcse.
From www.sliderbase.com
Properties of Water Presentation Biology Why Are Ionic Compounds Soluble In Water Gcse See the study guide on the three. However, they often dissolve in water to form an aqueous solution. Most ionic compounds are soluble in water and form aqueous solutions. At room temperature, ionic compounds are typically in a solid state. Ionic compounds have high melting and boiling points due to the strong forces of attraction between the ions. Close aqueous. Why Are Ionic Compounds Soluble In Water Gcse.
From www.vrogue.co
What Is Solubility Definition Solubility Product Fact vrogue.co Why Are Ionic Compounds Soluble In Water Gcse Ionic compounds have high melting and boiling points due to the strong forces of attraction between the ions. See the study guide on the three. Close aqueous solution a solution in which the. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. However, they often dissolve in water to form an. Why Are Ionic Compounds Soluble In Water Gcse.
From www.slideserve.com
PPT Bonding PowerPoint Presentation ID3050946 Why Are Ionic Compounds Soluble In Water Gcse Polar solvent h 2 o dissociates the ionic compound by interacting with the charged ions and pulls them with a strong force of attraction. However, they often dissolve in water to form an aqueous solution. Ionic compounds have high melting and boiling points due to the strong forces of attraction between the ions. When ionic compounds dissolve in water, the. Why Are Ionic Compounds Soluble In Water Gcse.
From www.slideserve.com
PPT Chapter 5 Molecular View of Reactions in Aqueous Solutions Part I Why Are Ionic Compounds Soluble In Water Gcse However, they often dissolve in water to form an aqueous solution. Most ionic compounds are soluble in water and form aqueous solutions. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Ionic compounds are good conductors of electricity in the molten state or in solution when the ionic compound is melted. Why Are Ionic Compounds Soluble In Water Gcse.
From study.com
Compound Solubility in Water Overview & Examples Lesson Why Are Ionic Compounds Soluble In Water Gcse Most ionic compounds are soluble in water and form aqueous solutions. However, they often dissolve in water to form an aqueous solution. At room temperature, ionic compounds are typically in a solid state. Polar solvent h 2 o dissociates the ionic compound by interacting with the charged ions and pulls them with a strong force of attraction. When ionic compounds. Why Are Ionic Compounds Soluble In Water Gcse.
From www.slideserve.com
PPT With enough water molecules, a soluble ionic compound is Why Are Ionic Compounds Soluble In Water Gcse When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. At room temperature, ionic compounds are typically in a solid state. Polar solvent h 2 o dissociates the ionic compound by interacting with. Why Are Ionic Compounds Soluble In Water Gcse.
From www.slideserve.com
PPT Reaction Stoichiometry Mole Method Calculations PowerPoint Why Are Ionic Compounds Soluble In Water Gcse However, they often dissolve in water to form an aqueous solution. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. Ionic compounds are good conductors of electricity in the molten state or in solution when the ionic compound is melted or dissolved in. Polar solvent h 2 o dissociates the. Why Are Ionic Compounds Soluble In Water Gcse.
From www.chegg.com
Solved Some solubility rules for ionic compounds in water Why Are Ionic Compounds Soluble In Water Gcse See the study guide on the three. At room temperature, ionic compounds are typically in a solid state. Close aqueous solution a solution in which the. However, they often dissolve in water to form an aqueous solution. Ionic compounds have high melting and boiling points due to the strong forces of attraction between the ions. Polar solvent h 2 o. Why Are Ionic Compounds Soluble In Water Gcse.
From www.numerade.com
SOLVEDWhy are the ionic compounds soluble in water? Why Are Ionic Compounds Soluble In Water Gcse Most ionic compounds are soluble in water and form aqueous solutions. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. At room temperature, ionic compounds are typically in a solid state. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. Polar. Why Are Ionic Compounds Soluble In Water Gcse.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID6635190 Why Are Ionic Compounds Soluble In Water Gcse When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. Most ionic compounds are soluble in water and form aqueous solutions. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Polar solvent h 2 o dissociates the ionic compound by interacting with. Why Are Ionic Compounds Soluble In Water Gcse.
From www.slideserve.com
PPT Solution Chemistry PowerPoint Presentation, free download ID Why Are Ionic Compounds Soluble In Water Gcse Most ionic compounds are soluble in water and form aqueous solutions. Ionic compounds are good conductors of electricity in the molten state or in solution when the ionic compound is melted or dissolved in. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Close aqueous solution a solution in which the.. Why Are Ionic Compounds Soluble In Water Gcse.
From www.youtube.com
Predicting solubility of ionic compounds in water YouTube Why Are Ionic Compounds Soluble In Water Gcse At room temperature, ionic compounds are typically in a solid state. Polar solvent h 2 o dissociates the ionic compound by interacting with the charged ions and pulls them with a strong force of attraction. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. However, they often dissolve in water. Why Are Ionic Compounds Soluble In Water Gcse.
From www.slideserve.com
PPT With enough water molecules, a soluble ionic compound is Why Are Ionic Compounds Soluble In Water Gcse At room temperature, ionic compounds are typically in a solid state. Ionic compounds are good conductors of electricity in the molten state or in solution when the ionic compound is melted or dissolved in. See the study guide on the three. Most ionic compounds are soluble in water and form aqueous solutions. Ionic compounds have high melting and boiling points,. Why Are Ionic Compounds Soluble In Water Gcse.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation ID2276550 Why Are Ionic Compounds Soluble In Water Gcse Close aqueous solution a solution in which the. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. Ionic compounds are good conductors of electricity in the molten state or in solution when the ionic compound is melted or dissolved in. Polar solvent h 2 o dissociates the ionic compound by. Why Are Ionic Compounds Soluble In Water Gcse.
From www.chemistryspace.com
Solubility Rules for Ionic Compounds Why Are Ionic Compounds Soluble In Water Gcse Most ionic compounds are soluble in water and form aqueous solutions. Ionic compounds have high melting and boiling points due to the strong forces of attraction between the ions. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. See the study guide on the three. At room temperature, ionic compounds are. Why Are Ionic Compounds Soluble In Water Gcse.
From www.chegg.com
Solved Are all ionic compounds soluble in water? Why Are Ionic Compounds Soluble In Water Gcse Polar solvent h 2 o dissociates the ionic compound by interacting with the charged ions and pulls them with a strong force of attraction. See the study guide on the three. However, they often dissolve in water to form an aqueous solution. At room temperature, ionic compounds are typically in a solid state. When ionic compounds dissolve in water, the. Why Are Ionic Compounds Soluble In Water Gcse.
From slidetodoc.com
Chapter 8 Section 2 Solubility 2 WaterThe Universal Why Are Ionic Compounds Soluble In Water Gcse See the study guide on the three. Ionic compounds have high melting and boiling points due to the strong forces of attraction between the ions. However, they often dissolve in water to form an aqueous solution. Ionic compounds are good conductors of electricity in the molten state or in solution when the ionic compound is melted or dissolved in. When. Why Are Ionic Compounds Soluble In Water Gcse.
From studymind.co.uk
Ionic Compound Properties (GCSE Chemistry) Study Mind Why Are Ionic Compounds Soluble In Water Gcse Close aqueous solution a solution in which the. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. Ionic compounds are good conductors of electricity in the molten state or in solution when the ionic compound is melted or dissolved in. Polar solvent h 2 o dissociates the ionic compound by. Why Are Ionic Compounds Soluble In Water Gcse.
From www.chegg.com
Solved 3. Why are some ionic compounds soluble in water and Why Are Ionic Compounds Soluble In Water Gcse At room temperature, ionic compounds are typically in a solid state. Polar solvent h 2 o dissociates the ionic compound by interacting with the charged ions and pulls them with a strong force of attraction. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Ionic compounds have high melting and boiling. Why Are Ionic Compounds Soluble In Water Gcse.
From smallbusinessron.web.fc2.com
do covalent compounds dissolve in water Why Are Ionic Compounds Soluble In Water Gcse At room temperature, ionic compounds are typically in a solid state. However, they often dissolve in water to form an aqueous solution. Ionic compounds have high melting and boiling points due to the strong forces of attraction between the ions. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Most ionic. Why Are Ionic Compounds Soluble In Water Gcse.
From www.youtube.com
why ionic compounds are soluble in water? ( A proper reason ) YouTube Why Are Ionic Compounds Soluble In Water Gcse When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. At room temperature, ionic compounds are typically in a solid state. See the study guide on the three. Ionic compounds are good conductors of electricity in the molten state or in solution when the ionic compound is melted or dissolved in.. Why Are Ionic Compounds Soluble In Water Gcse.
From www.numerade.com
SOLVED Solubility Rulesfor some ionic compounds in water Soluble Ionic Why Are Ionic Compounds Soluble In Water Gcse See the study guide on the three. Close aqueous solution a solution in which the. Polar solvent h 2 o dissociates the ionic compound by interacting with the charged ions and pulls them with a strong force of attraction. Ionic compounds have high melting and boiling points due to the strong forces of attraction between the ions. Ionic compounds have. Why Are Ionic Compounds Soluble In Water Gcse.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Why Are Ionic Compounds Soluble In Water Gcse When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. At room temperature, ionic compounds are typically in a solid state. Ionic compounds are good conductors of electricity in the molten state or in solution when the ionic compound is melted or dissolved in. Ionic compounds have high melting and boiling. Why Are Ionic Compounds Soluble In Water Gcse.
From www.numerade.com
SOLVED Predict whether each of the following ionic compounds is Why Are Ionic Compounds Soluble In Water Gcse Ionic compounds are good conductors of electricity in the molten state or in solution when the ionic compound is melted or dissolved in. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Close aqueous solution a solution in which the. See the study guide on the three. However, they often dissolve. Why Are Ionic Compounds Soluble In Water Gcse.
From www.chegg.com
Solved Some solubility rules for ionic compounds in water Why Are Ionic Compounds Soluble In Water Gcse At room temperature, ionic compounds are typically in a solid state. Ionic compounds have high melting and boiling points due to the strong forces of attraction between the ions. Close aqueous solution a solution in which the. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. However, they often dissolve. Why Are Ionic Compounds Soluble In Water Gcse.
From www.hanlin.com
Edexcel IGCSE Chemistry 复习笔记 1.6 5 Ionic compounds Bonds, Structure Why Are Ionic Compounds Soluble In Water Gcse Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. At room temperature, ionic compounds are typically in a solid state. Close aqueous solution a solution in which the. See the study guide on the three. Ionic compounds are good conductors of electricity in the molten state or in solution when the. Why Are Ionic Compounds Soluble In Water Gcse.
From www.slideserve.com
PPT Chapter 7 Reactions in Aqueous Solutions PowerPoint Presentation Why Are Ionic Compounds Soluble In Water Gcse Ionic compounds have high melting and boiling points due to the strong forces of attraction between the ions. Most ionic compounds are soluble in water and form aqueous solutions. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Polar solvent h 2 o dissociates the ionic compound by interacting with the. Why Are Ionic Compounds Soluble In Water Gcse.
From chemcollective.org
CHEM 1315 Lab 10 Conservation of Mass Why Are Ionic Compounds Soluble In Water Gcse Ionic compounds are good conductors of electricity in the molten state or in solution when the ionic compound is melted or dissolved in. However, they often dissolve in water to form an aqueous solution. Most ionic compounds are soluble in water and form aqueous solutions. See the study guide on the three. Polar solvent h 2 o dissociates the ionic. Why Are Ionic Compounds Soluble In Water Gcse.
From www.slideserve.com
PPT With enough water molecules, a soluble ionic compound is Why Are Ionic Compounds Soluble In Water Gcse At room temperature, ionic compounds are typically in a solid state. Most ionic compounds are soluble in water and form aqueous solutions. See the study guide on the three. Polar solvent h 2 o dissociates the ionic compound by interacting with the charged ions and pulls them with a strong force of attraction. Ionic compounds have high melting and boiling. Why Are Ionic Compounds Soluble In Water Gcse.
From kunduz.com
[ANSWERED] Which of the following ionic compounds is soluble in water Why Are Ionic Compounds Soluble In Water Gcse When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. However, they often dissolve in water to form an aqueous solution. At room temperature, ionic compounds are typically in a solid state. Close aqueous solution a solution in which the. Most ionic compounds are soluble in water and form aqueous solutions.. Why Are Ionic Compounds Soluble In Water Gcse.
From www.ck12.org
Physical Properties of Ionic Compounds ( Read ) Chemistry CK12 Why Are Ionic Compounds Soluble In Water Gcse Most ionic compounds are soluble in water and form aqueous solutions. Close aqueous solution a solution in which the. Polar solvent h 2 o dissociates the ionic compound by interacting with the charged ions and pulls them with a strong force of attraction. At room temperature, ionic compounds are typically in a solid state. Ionic compounds are good conductors of. Why Are Ionic Compounds Soluble In Water Gcse.