Potassium Bromide And Chlorine Water Equation . cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. The aqueous layer contains a mixture of. Revision notes on 2.3.2 halogen displacement reactions for the edexcel a. Hexanes are added to form two. Cl 2 (g) + 2kbr(aq) → br 2 (aq) +. Chlorine + potassium bromide → bromine + potassium chloride: chlorine is bubbled through the potassium bromide layer. The slideshow shows what happens when solutions. — reaction between chlorine water and potassium bromide solutionform 5 chemistry kssm chapter 1 redox. The chlorine reacts with the bromide to form bromine. cl + kbr = kcl + br is a single displacement (substitution) reaction where one mole of chlorine [cl] gas and one mole of aqueous.
from www.numerade.com
Chlorine + potassium bromide → bromine + potassium chloride: Revision notes on 2.3.2 halogen displacement reactions for the edexcel a. — reaction between chlorine water and potassium bromide solutionform 5 chemistry kssm chapter 1 redox. cl + kbr = kcl + br is a single displacement (substitution) reaction where one mole of chlorine [cl] gas and one mole of aqueous. The aqueous layer contains a mixture of. Cl 2 (g) + 2kbr(aq) → br 2 (aq) +. Hexanes are added to form two. The slideshow shows what happens when solutions. The chlorine reacts with the bromide to form bromine. chlorine is bubbled through the potassium bromide layer.
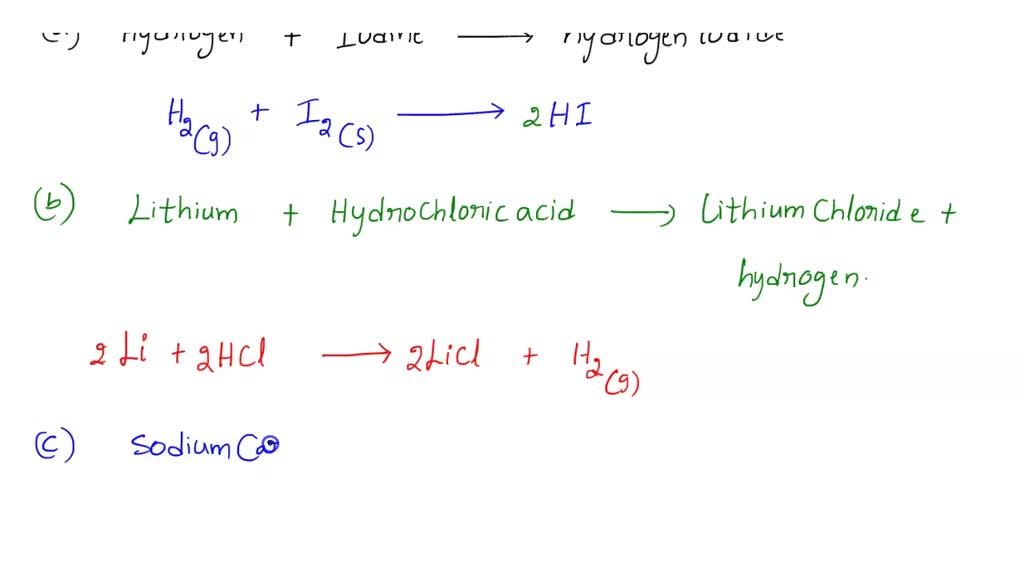
SOLVED BALANCING CHEMICAL EQUATIONS HYDROGEN GAS OXYGEN GAS WATER SODIUM SOLID IODINE SOLID
Potassium Bromide And Chlorine Water Equation — reaction between chlorine water and potassium bromide solutionform 5 chemistry kssm chapter 1 redox. — reaction between chlorine water and potassium bromide solutionform 5 chemistry kssm chapter 1 redox. The aqueous layer contains a mixture of. The chlorine reacts with the bromide to form bromine. Chlorine + potassium bromide → bromine + potassium chloride: cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. The slideshow shows what happens when solutions. Hexanes are added to form two. Revision notes on 2.3.2 halogen displacement reactions for the edexcel a. chlorine is bubbled through the potassium bromide layer. Cl 2 (g) + 2kbr(aq) → br 2 (aq) +. cl + kbr = kcl + br is a single displacement (substitution) reaction where one mole of chlorine [cl] gas and one mole of aqueous.
From gbu-presnenskij.ru
What Is The Ionic Equation For The Reaction Of Chlorine, 59 OFF Potassium Bromide And Chlorine Water Equation — reaction between chlorine water and potassium bromide solutionform 5 chemistry kssm chapter 1 redox. Revision notes on 2.3.2 halogen displacement reactions for the edexcel a. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Cl 2 (g) + 2kbr(aq) → br 2 (aq) +.. Potassium Bromide And Chlorine Water Equation.
From www.slideshare.net
Reactions Of The Halogens (2) Potassium Bromide And Chlorine Water Equation The aqueous layer contains a mixture of. chlorine is bubbled through the potassium bromide layer. Revision notes on 2.3.2 halogen displacement reactions for the edexcel a. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Cl 2 (g) + 2kbr(aq) → br 2 (aq) +.. Potassium Bromide And Chlorine Water Equation.
From www.slideshare.net
Lesson 2 Halide Halogen Displacement Reactions. Potassium Bromide And Chlorine Water Equation cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. — reaction between chlorine water and potassium bromide solutionform 5 chemistry kssm chapter 1 redox. The aqueous layer contains a mixture of. The chlorine reacts with the bromide to form bromine. Hexanes are added to form. Potassium Bromide And Chlorine Water Equation.
From www.numerade.com
SOLVEDA mixture of potassium chloride and potassium bromide weighing 3.595 g is heated with Potassium Bromide And Chlorine Water Equation cl + kbr = kcl + br is a single displacement (substitution) reaction where one mole of chlorine [cl] gas and one mole of aqueous. Hexanes are added to form two. Chlorine + potassium bromide → bromine + potassium chloride: The chlorine reacts with the bromide to form bromine. — reaction between chlorine water and potassium bromide solutionform. Potassium Bromide And Chlorine Water Equation.
From www.bhphotovideo.com
Photographers' Formulary Potassium Bromide (100g) 100930 100G Potassium Bromide And Chlorine Water Equation Revision notes on 2.3.2 halogen displacement reactions for the edexcel a. The aqueous layer contains a mixture of. chlorine is bubbled through the potassium bromide layer. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. The slideshow shows what happens when solutions. Chlorine + potassium. Potassium Bromide And Chlorine Water Equation.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID2110165 Potassium Bromide And Chlorine Water Equation cl + kbr = kcl + br is a single displacement (substitution) reaction where one mole of chlorine [cl] gas and one mole of aqueous. Revision notes on 2.3.2 halogen displacement reactions for the edexcel a. The slideshow shows what happens when solutions. The aqueous layer contains a mixture of. Cl 2 (g) + 2kbr(aq) → br 2 (aq). Potassium Bromide And Chlorine Water Equation.
From www.youtube.com
Reaction 2 Potassium Bromide and Chlorine Water YouTube Potassium Bromide And Chlorine Water Equation The chlorine reacts with the bromide to form bromine. — reaction between chlorine water and potassium bromide solutionform 5 chemistry kssm chapter 1 redox. Revision notes on 2.3.2 halogen displacement reactions for the edexcel a. Hexanes are added to form two. chlorine is bubbled through the potassium bromide layer. Chlorine + potassium bromide → bromine + potassium chloride:. Potassium Bromide And Chlorine Water Equation.
From www.nagwa.com
Question Video Identifying the Chemical Equation That Describes the Reaction between Potassium Potassium Bromide And Chlorine Water Equation The chlorine reacts with the bromide to form bromine. chlorine is bubbled through the potassium bromide layer. The aqueous layer contains a mixture of. Revision notes on 2.3.2 halogen displacement reactions for the edexcel a. — reaction between chlorine water and potassium bromide solutionform 5 chemistry kssm chapter 1 redox. The slideshow shows what happens when solutions. Hexanes. Potassium Bromide And Chlorine Water Equation.
From www.slideserve.com
PPT Explain, in terms of electrons, why potassium reacts more violently than sodium. (3 marks Potassium Bromide And Chlorine Water Equation Chlorine + potassium bromide → bromine + potassium chloride: cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. cl + kbr = kcl + br is a single displacement (substitution) reaction where one mole of chlorine [cl] gas and one mole of aqueous. Hexanes are. Potassium Bromide And Chlorine Water Equation.
From www.chemkits.eu
Sodium bromide, 99.8+, 7647156 Potassium Bromide And Chlorine Water Equation The slideshow shows what happens when solutions. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Revision notes on 2.3.2 halogen displacement reactions for the edexcel a. — reaction between chlorine water and potassium bromide solutionform 5 chemistry kssm chapter 1 redox. Cl 2 (g). Potassium Bromide And Chlorine Water Equation.
From socratic.org
What is the balanced chemical equation for this reaction Gaseous chlorine reacts with an Potassium Bromide And Chlorine Water Equation cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. The aqueous layer contains a mixture of. Revision notes on 2.3.2 halogen displacement reactions for the edexcel a. Chlorine + potassium bromide → bromine + potassium chloride: Hexanes are added to form two. Cl 2 (g) +. Potassium Bromide And Chlorine Water Equation.
From www.numerade.com
SOLVED Metals bond with halogens to form colorless metal halides. During an experiment Potassium Bromide And Chlorine Water Equation Revision notes on 2.3.2 halogen displacement reactions for the edexcel a. chlorine is bubbled through the potassium bromide layer. The chlorine reacts with the bromide to form bromine. The aqueous layer contains a mixture of. Chlorine + potassium bromide → bromine + potassium chloride: Cl 2 (g) + 2kbr(aq) → br 2 (aq) +. — reaction between chlorine. Potassium Bromide And Chlorine Water Equation.
From us.metoree.com
41 Ethyl Bromide Manufacturers in 2024 Metoree Potassium Bromide And Chlorine Water Equation — reaction between chlorine water and potassium bromide solutionform 5 chemistry kssm chapter 1 redox. The aqueous layer contains a mixture of. Chlorine + potassium bromide → bromine + potassium chloride: Cl 2 (g) + 2kbr(aq) → br 2 (aq) +. Hexanes are added to form two. Revision notes on 2.3.2 halogen displacement reactions for the edexcel a. The. Potassium Bromide And Chlorine Water Equation.
From www.thesciencehive.co.uk
Group7halogensanswers — the science sauce Potassium Bromide And Chlorine Water Equation cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Hexanes are added to form two. — reaction between chlorine water and potassium bromide solutionform 5 chemistry kssm chapter 1 redox. cl + kbr = kcl + br is a single displacement (substitution) reaction where. Potassium Bromide And Chlorine Water Equation.
From www.youtube.com
Chlorine And Sodium Bromide Make Sodium Chloride And Bromine YouTube Potassium Bromide And Chlorine Water Equation The slideshow shows what happens when solutions. chlorine is bubbled through the potassium bromide layer. The chlorine reacts with the bromide to form bromine. Revision notes on 2.3.2 halogen displacement reactions for the edexcel a. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Hexanes. Potassium Bromide And Chlorine Water Equation.
From www.chemistryworld.com
Potassium bromide Podcast Chemistry World Potassium Bromide And Chlorine Water Equation cl + kbr = kcl + br is a single displacement (substitution) reaction where one mole of chlorine [cl] gas and one mole of aqueous. The aqueous layer contains a mixture of. Hexanes are added to form two. Cl 2 (g) + 2kbr(aq) → br 2 (aq) +. The chlorine reacts with the bromide to form bromine. Revision notes. Potassium Bromide And Chlorine Water Equation.
From www.rpicorp.com
E718001.0 Ethidium Bromide, Powder, 1 Gram Potassium Bromide And Chlorine Water Equation cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. — reaction between chlorine water and potassium bromide solutionform 5 chemistry kssm chapter 1 redox. Revision notes on 2.3.2 halogen displacement reactions for the edexcel a. Chlorine + potassium bromide → bromine + potassium chloride: Cl. Potassium Bromide And Chlorine Water Equation.
From www.rpicorp.com
E718005.0 Ethidium Bromide, Powder, 5 Grams Potassium Bromide And Chlorine Water Equation The slideshow shows what happens when solutions. The chlorine reacts with the bromide to form bromine. — reaction between chlorine water and potassium bromide solutionform 5 chemistry kssm chapter 1 redox. The aqueous layer contains a mixture of. cl + kbr = kcl + br is a single displacement (substitution) reaction where one mole of chlorine [cl] gas. Potassium Bromide And Chlorine Water Equation.
From www.toppr.com
In potassium bromide solution, chlorine displace Potassium Bromide And Chlorine Water Equation Chlorine + potassium bromide → bromine + potassium chloride: The aqueous layer contains a mixture of. chlorine is bubbled through the potassium bromide layer. The slideshow shows what happens when solutions. Cl 2 (g) + 2kbr(aq) → br 2 (aq) +. Revision notes on 2.3.2 halogen displacement reactions for the edexcel a. — reaction between chlorine water and. Potassium Bromide And Chlorine Water Equation.
From www.youtube.com
How to Balance KBr + Cl2 = KCl + Br2 (Potassium bromide + Chlorine gas) YouTube Potassium Bromide And Chlorine Water Equation chlorine is bubbled through the potassium bromide layer. The slideshow shows what happens when solutions. The aqueous layer contains a mixture of. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. The chlorine reacts with the bromide to form bromine. Cl 2 (g) + 2kbr(aq). Potassium Bromide And Chlorine Water Equation.
From www.dreamstime.com
Potassium Bromide Chemical Formula on Waterdrop Background Stock Illustration Illustration of Potassium Bromide And Chlorine Water Equation The slideshow shows what happens when solutions. Hexanes are added to form two. The chlorine reacts with the bromide to form bromine. — reaction between chlorine water and potassium bromide solutionform 5 chemistry kssm chapter 1 redox. cl + kbr = kcl + br is a single displacement (substitution) reaction where one mole of chlorine [cl] gas and. Potassium Bromide And Chlorine Water Equation.
From www.macsenlab.com
Pinaverium Bromide API 53251948 Manufacturer & Supplier Potassium Bromide And Chlorine Water Equation The aqueous layer contains a mixture of. The slideshow shows what happens when solutions. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. — reaction between chlorine water and potassium bromide solutionform 5 chemistry kssm chapter 1 redox. cl + kbr = kcl +. Potassium Bromide And Chlorine Water Equation.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine molecule, and (c) a bromide ion Potassium Bromide And Chlorine Water Equation Chlorine + potassium bromide → bromine + potassium chloride: The slideshow shows what happens when solutions. — reaction between chlorine water and potassium bromide solutionform 5 chemistry kssm chapter 1 redox. The chlorine reacts with the bromide to form bromine. Hexanes are added to form two. Cl 2 (g) + 2kbr(aq) → br 2 (aq) +. cl 2. Potassium Bromide And Chlorine Water Equation.
From www.youtube.com
How to Write the Formula for Potassium bromate YouTube Potassium Bromide And Chlorine Water Equation The chlorine reacts with the bromide to form bromine. Cl 2 (g) + 2kbr(aq) → br 2 (aq) +. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Hexanes are added to form two. — reaction between chlorine water and potassium bromide solutionform 5 chemistry. Potassium Bromide And Chlorine Water Equation.
From www.slideshare.net
Chemical Reactions Potassium Bromide And Chlorine Water Equation chlorine is bubbled through the potassium bromide layer. — reaction between chlorine water and potassium bromide solutionform 5 chemistry kssm chapter 1 redox. The aqueous layer contains a mixture of. The slideshow shows what happens when solutions. Chlorine + potassium bromide → bromine + potassium chloride: Revision notes on 2.3.2 halogen displacement reactions for the edexcel a. Hexanes. Potassium Bromide And Chlorine Water Equation.
From www.youtube.com
Equation for KBr + H2O (Potassium bromide + Water) YouTube Potassium Bromide And Chlorine Water Equation cl + kbr = kcl + br is a single displacement (substitution) reaction where one mole of chlorine [cl] gas and one mole of aqueous. Revision notes on 2.3.2 halogen displacement reactions for the edexcel a. The chlorine reacts with the bromide to form bromine. The aqueous layer contains a mixture of. Cl 2 (g) + 2kbr(aq) → br. Potassium Bromide And Chlorine Water Equation.
From www.numerade.com
SOLVED BALANCING CHEMICAL EQUATIONS HYDROGEN GAS OXYGEN GAS WATER SODIUM SOLID IODINE SOLID Potassium Bromide And Chlorine Water Equation Hexanes are added to form two. Revision notes on 2.3.2 halogen displacement reactions for the edexcel a. The chlorine reacts with the bromide to form bromine. Cl 2 (g) + 2kbr(aq) → br 2 (aq) +. — reaction between chlorine water and potassium bromide solutionform 5 chemistry kssm chapter 1 redox. Chlorine + potassium bromide → bromine + potassium. Potassium Bromide And Chlorine Water Equation.
From exontsuic.blob.core.windows.net
Chlorine Gas Balanced Equation at Thomas Sheehan blog Potassium Bromide And Chlorine Water Equation cl + kbr = kcl + br is a single displacement (substitution) reaction where one mole of chlorine [cl] gas and one mole of aqueous. The chlorine reacts with the bromide to form bromine. The aqueous layer contains a mixture of. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns. Potassium Bromide And Chlorine Water Equation.
From www.shutterstock.com
Sodium Bromide Properties Chemical Compound Structure Stock Vector (Royalty Free) 1977753731 Potassium Bromide And Chlorine Water Equation The chlorine reacts with the bromide to form bromine. Revision notes on 2.3.2 halogen displacement reactions for the edexcel a. Chlorine + potassium bromide → bromine + potassium chloride: Cl 2 (g) + 2kbr(aq) → br 2 (aq) +. Hexanes are added to form two. The aqueous layer contains a mixture of. The slideshow shows what happens when solutions. . Potassium Bromide And Chlorine Water Equation.
From www.grainger.com
7758023, 119, Potassium Bromide, Crystal, Reagent, ACS 6MMU1P1220500GM Grainger Potassium Bromide And Chlorine Water Equation The chlorine reacts with the bromide to form bromine. Cl 2 (g) + 2kbr(aq) → br 2 (aq) +. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. chlorine is bubbled through the potassium bromide layer. The aqueous layer contains a mixture of. Revision notes. Potassium Bromide And Chlorine Water Equation.
From brainly.in
Chlorine reacts with potassium bromide. complete the word equation Brainly.in Potassium Bromide And Chlorine Water Equation Revision notes on 2.3.2 halogen displacement reactions for the edexcel a. cl + kbr = kcl + br is a single displacement (substitution) reaction where one mole of chlorine [cl] gas and one mole of aqueous. Chlorine + potassium bromide → bromine + potassium chloride: The slideshow shows what happens when solutions. Cl 2 (g) + 2kbr(aq) → br. Potassium Bromide And Chlorine Water Equation.
From www.dreamstime.com
3D Image of Vinyl Bromide Skeletal Formula Stock Illustration Illustration of stuff, alcohol Potassium Bromide And Chlorine Water Equation Chlorine + potassium bromide → bromine + potassium chloride: The slideshow shows what happens when solutions. Hexanes are added to form two. — reaction between chlorine water and potassium bromide solutionform 5 chemistry kssm chapter 1 redox. Revision notes on 2.3.2 halogen displacement reactions for the edexcel a. chlorine is bubbled through the potassium bromide layer. cl. Potassium Bromide And Chlorine Water Equation.
From slideplayer.com
How do you know how many atoms and how many elements are in a symbol equation? ppt download Potassium Bromide And Chlorine Water Equation The aqueous layer contains a mixture of. — reaction between chlorine water and potassium bromide solutionform 5 chemistry kssm chapter 1 redox. cl + kbr = kcl + br is a single displacement (substitution) reaction where one mole of chlorine [cl] gas and one mole of aqueous. The slideshow shows what happens when solutions. The chlorine reacts with. Potassium Bromide And Chlorine Water Equation.
From www.chemkits.eu
Potassium bromide, 98.5, 7758023 Potassium Bromide And Chlorine Water Equation — reaction between chlorine water and potassium bromide solutionform 5 chemistry kssm chapter 1 redox. cl + kbr = kcl + br is a single displacement (substitution) reaction where one mole of chlorine [cl] gas and one mole of aqueous. chlorine is bubbled through the potassium bromide layer. The slideshow shows what happens when solutions. Revision notes. Potassium Bromide And Chlorine Water Equation.
From www.numerade.com
SOLVEDGaseous chlorine will displace bromide ion from an aqueous solution of potassium bromide Potassium Bromide And Chlorine Water Equation Chlorine + potassium bromide → bromine + potassium chloride: — reaction between chlorine water and potassium bromide solutionform 5 chemistry kssm chapter 1 redox. cl + kbr = kcl + br is a single displacement (substitution) reaction where one mole of chlorine [cl] gas and one mole of aqueous. Cl 2 (g) + 2kbr(aq) → br 2 (aq). Potassium Bromide And Chlorine Water Equation.