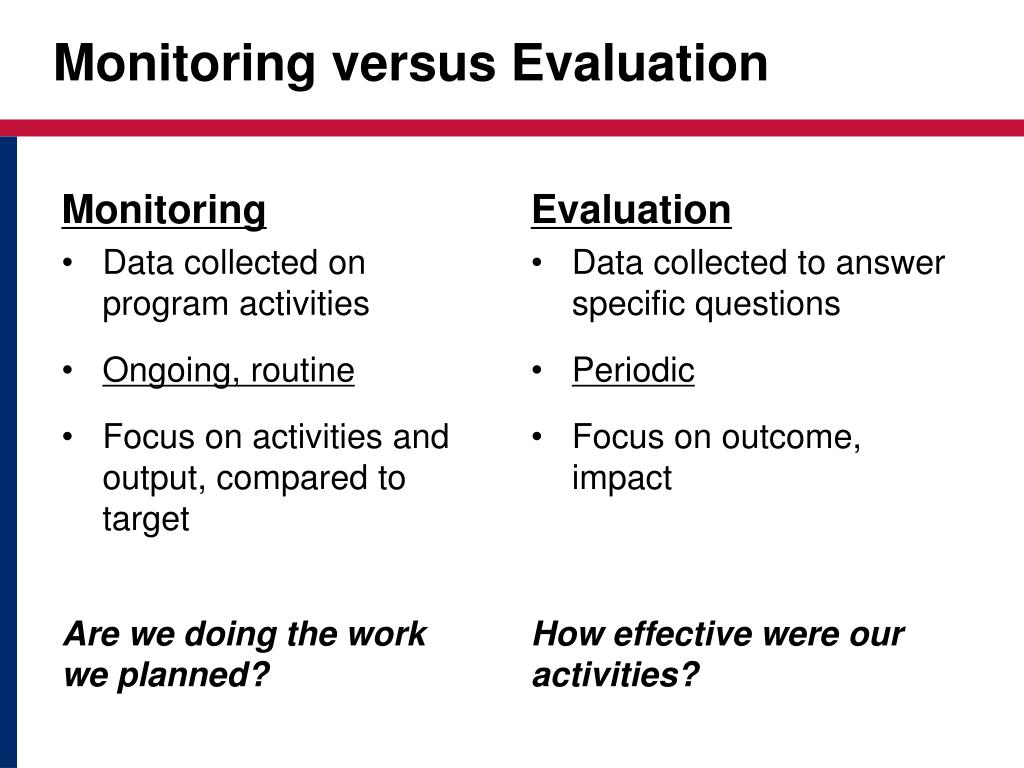
Monitoring Study Definition . Monitoring visits are an essential component of any clinical research project. This article discusses the principles and practice of monitoring and evaluation and emphasises that monitoring and evaluation (m. Monitoring ensure that a specific public health program to prevent and or stop epidemic is carried out as planned so that the goal and objectives are meet. It contains the e6 (r2) addenda, and provides an overview of the scope of, requirements to clinical trial monitoring process, as well as. Monitoring is defined as the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded, and reported in. Our first three principles link with parts a, b and c of the ich. They help the sponsor and the clinical research associate (cra) confirm the integrity of the study data as.
from www.slideserve.com
This article discusses the principles and practice of monitoring and evaluation and emphasises that monitoring and evaluation (m. It contains the e6 (r2) addenda, and provides an overview of the scope of, requirements to clinical trial monitoring process, as well as. Monitoring visits are an essential component of any clinical research project. Monitoring is defined as the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded, and reported in. They help the sponsor and the clinical research associate (cra) confirm the integrity of the study data as. Our first three principles link with parts a, b and c of the ich. Monitoring ensure that a specific public health program to prevent and or stop epidemic is carried out as planned so that the goal and objectives are meet.
PPT Key Terms for Monitoring and Evaluation PowerPoint Presentation
Monitoring Study Definition Our first three principles link with parts a, b and c of the ich. They help the sponsor and the clinical research associate (cra) confirm the integrity of the study data as. It contains the e6 (r2) addenda, and provides an overview of the scope of, requirements to clinical trial monitoring process, as well as. Monitoring visits are an essential component of any clinical research project. Monitoring is defined as the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded, and reported in. Monitoring ensure that a specific public health program to prevent and or stop epidemic is carried out as planned so that the goal and objectives are meet. Our first three principles link with parts a, b and c of the ich. This article discusses the principles and practice of monitoring and evaluation and emphasises that monitoring and evaluation (m.
From www.atatus.com
Continuous Monitoring Definition, Types, Benefits and More Monitoring Study Definition This article discusses the principles and practice of monitoring and evaluation and emphasises that monitoring and evaluation (m. It contains the e6 (r2) addenda, and provides an overview of the scope of, requirements to clinical trial monitoring process, as well as. Monitoring visits are an essential component of any clinical research project. Monitoring is defined as the act of overseeing. Monitoring Study Definition.
From www.eslactivity.org
Monitoring ESL Learners How and Why to Monitor Learners Monitoring Study Definition It contains the e6 (r2) addenda, and provides an overview of the scope of, requirements to clinical trial monitoring process, as well as. Monitoring visits are an essential component of any clinical research project. They help the sponsor and the clinical research associate (cra) confirm the integrity of the study data as. Monitoring ensure that a specific public health program. Monitoring Study Definition.
From www.youtube.com
Whats the difference between Monitoring and Evaluation? YouTube Monitoring Study Definition Monitoring visits are an essential component of any clinical research project. It contains the e6 (r2) addenda, and provides an overview of the scope of, requirements to clinical trial monitoring process, as well as. Our first three principles link with parts a, b and c of the ich. Monitoring is defined as the act of overseeing the progress of a. Monitoring Study Definition.
From wirtschaftslexikon.gabler.de
Monitoring • Definition Gabler Wirtschaftslexikon Monitoring Study Definition It contains the e6 (r2) addenda, and provides an overview of the scope of, requirements to clinical trial monitoring process, as well as. They help the sponsor and the clinical research associate (cra) confirm the integrity of the study data as. Monitoring visits are an essential component of any clinical research project. This article discusses the principles and practice of. Monitoring Study Definition.
From www.youtube.com
What is Monitor Definition of Monitor What is computer monitor Monitoring Study Definition Monitoring visits are an essential component of any clinical research project. They help the sponsor and the clinical research associate (cra) confirm the integrity of the study data as. Monitoring is defined as the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded, and reported in. It contains the e6 (r2) addenda,. Monitoring Study Definition.
From www.slideserve.com
PPT Tips to a Successful Monitoring Visit PowerPoint Presentation Monitoring Study Definition This article discusses the principles and practice of monitoring and evaluation and emphasises that monitoring and evaluation (m. It contains the e6 (r2) addenda, and provides an overview of the scope of, requirements to clinical trial monitoring process, as well as. Monitoring is defined as the act of overseeing the progress of a clinical trial, and of ensuring that it. Monitoring Study Definition.
From www.letsgolearn.com
Progress Monitoring and Formative Assessment Let's Go Learn Monitoring Study Definition They help the sponsor and the clinical research associate (cra) confirm the integrity of the study data as. Monitoring is defined as the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded, and reported in. Our first three principles link with parts a, b and c of the ich. It contains the. Monitoring Study Definition.
From www.slideserve.com
PPT Introduction to Monitoring and Evaluation PowerPoint Presentation Monitoring Study Definition This article discusses the principles and practice of monitoring and evaluation and emphasises that monitoring and evaluation (m. They help the sponsor and the clinical research associate (cra) confirm the integrity of the study data as. Monitoring visits are an essential component of any clinical research project. Our first three principles link with parts a, b and c of the. Monitoring Study Definition.
From www.youtube.com
STRUCTURAL HEALTH MONITORING DEFINITION INTRODUCTION SCOPE Monitoring Study Definition It contains the e6 (r2) addenda, and provides an overview of the scope of, requirements to clinical trial monitoring process, as well as. Monitoring is defined as the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded, and reported in. Our first three principles link with parts a, b and c of. Monitoring Study Definition.
From study.com
SelfMonitoring in Psychology Definition, Theory & Examples Video Monitoring Study Definition Our first three principles link with parts a, b and c of the ich. Monitoring is defined as the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded, and reported in. This article discusses the principles and practice of monitoring and evaluation and emphasises that monitoring and evaluation (m. Monitoring ensure that. Monitoring Study Definition.
From www.slideserve.com
PPT Clinical Monitoring PowerPoint Presentation, free download ID Monitoring Study Definition They help the sponsor and the clinical research associate (cra) confirm the integrity of the study data as. It contains the e6 (r2) addenda, and provides an overview of the scope of, requirements to clinical trial monitoring process, as well as. Our first three principles link with parts a, b and c of the ich. Monitoring is defined as the. Monitoring Study Definition.
From studentprivacycompass.org
Understanding Student Monitoring Student Privacy Compass Monitoring Study Definition Monitoring visits are an essential component of any clinical research project. Monitoring is defined as the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded, and reported in. This article discusses the principles and practice of monitoring and evaluation and emphasises that monitoring and evaluation (m. Our first three principles link with. Monitoring Study Definition.
From eqcadvisory.com
Step 3 Monitor Reviews EQC Advisory Limited Monitoring Study Definition Our first three principles link with parts a, b and c of the ich. Monitoring visits are an essential component of any clinical research project. Monitoring is defined as the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded, and reported in. This article discusses the principles and practice of monitoring and. Monitoring Study Definition.
From www.giz.de
Monitoring giz.de Monitoring Study Definition Monitoring ensure that a specific public health program to prevent and or stop epidemic is carried out as planned so that the goal and objectives are meet. It contains the e6 (r2) addenda, and provides an overview of the scope of, requirements to clinical trial monitoring process, as well as. Monitoring visits are an essential component of any clinical research. Monitoring Study Definition.
From www.slideserve.com
PPT Module 2 Monitoring and Evaluation Definitions PowerPoint Monitoring Study Definition Monitoring ensure that a specific public health program to prevent and or stop epidemic is carried out as planned so that the goal and objectives are meet. Monitoring is defined as the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded, and reported in. It contains the e6 (r2) addenda, and provides. Monitoring Study Definition.
From slideplayer.com
SITS Monitoring Study SITSMOST ppt download Monitoring Study Definition Our first three principles link with parts a, b and c of the ich. This article discusses the principles and practice of monitoring and evaluation and emphasises that monitoring and evaluation (m. They help the sponsor and the clinical research associate (cra) confirm the integrity of the study data as. Monitoring ensure that a specific public health program to prevent. Monitoring Study Definition.
From progresslearning.com
Why Progress Monitoring is Critical for Supporting Students with IEPs Monitoring Study Definition This article discusses the principles and practice of monitoring and evaluation and emphasises that monitoring and evaluation (m. Monitoring ensure that a specific public health program to prevent and or stop epidemic is carried out as planned so that the goal and objectives are meet. Our first three principles link with parts a, b and c of the ich. They. Monitoring Study Definition.
From www.slideserve.com
PPT Study Monitoring What Does It Mean & How To Do It Right Monitoring Study Definition This article discusses the principles and practice of monitoring and evaluation and emphasises that monitoring and evaluation (m. Monitoring ensure that a specific public health program to prevent and or stop epidemic is carried out as planned so that the goal and objectives are meet. They help the sponsor and the clinical research associate (cra) confirm the integrity of the. Monitoring Study Definition.
From www.researchgate.net
Canvas monitoring diagram. The monitoring framework relies on a layer Monitoring Study Definition They help the sponsor and the clinical research associate (cra) confirm the integrity of the study data as. Monitoring is defined as the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded, and reported in. It contains the e6 (r2) addenda, and provides an overview of the scope of, requirements to clinical. Monitoring Study Definition.
From www.slideserve.com
PPT SelfMonitoring PowerPoint Presentation, free download ID2652680 Monitoring Study Definition Monitoring is defined as the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded, and reported in. It contains the e6 (r2) addenda, and provides an overview of the scope of, requirements to clinical trial monitoring process, as well as. They help the sponsor and the clinical research associate (cra) confirm the. Monitoring Study Definition.
From www.slideshare.net
Explaining the Different Types of Routine Monitoring Visits Monitoring Study Definition Monitoring is defined as the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded, and reported in. It contains the e6 (r2) addenda, and provides an overview of the scope of, requirements to clinical trial monitoring process, as well as. This article discusses the principles and practice of monitoring and evaluation and. Monitoring Study Definition.
From www.financestrategists.com
Investment Monitoring Definition, Process, & Key Components Monitoring Study Definition This article discusses the principles and practice of monitoring and evaluation and emphasises that monitoring and evaluation (m. Our first three principles link with parts a, b and c of the ich. Monitoring visits are an essential component of any clinical research project. It contains the e6 (r2) addenda, and provides an overview of the scope of, requirements to clinical. Monitoring Study Definition.
From www.slideserve.com
PPT Project Planning, Monitoring & Evaluation PowerPoint Presentation Monitoring Study Definition This article discusses the principles and practice of monitoring and evaluation and emphasises that monitoring and evaluation (m. It contains the e6 (r2) addenda, and provides an overview of the scope of, requirements to clinical trial monitoring process, as well as. Monitoring ensure that a specific public health program to prevent and or stop epidemic is carried out as planned. Monitoring Study Definition.
From medicinabasica.com
¿Qué es el estudio del monitor Holter? Medicina Básica Monitoring Study Definition Monitoring is defined as the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded, and reported in. This article discusses the principles and practice of monitoring and evaluation and emphasises that monitoring and evaluation (m. Monitoring visits are an essential component of any clinical research project. Monitoring ensure that a specific public. Monitoring Study Definition.
From laskarotomasi.com
Apa Itu Monitoring System dan Manfaatnya Bagi Perusahaan Monitoring Study Definition Monitoring is defined as the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded, and reported in. It contains the e6 (r2) addenda, and provides an overview of the scope of, requirements to clinical trial monitoring process, as well as. Monitoring visits are an essential component of any clinical research project. Monitoring. Monitoring Study Definition.
From view.genial.ly
6 Major Steps in Effective Progress Monitoring Monitoring Study Definition Our first three principles link with parts a, b and c of the ich. Monitoring is defined as the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded, and reported in. This article discusses the principles and practice of monitoring and evaluation and emphasises that monitoring and evaluation (m. It contains the. Monitoring Study Definition.
From ihararejobs.com
Key Reasons To Consider Studying Monitoring And Evaluation. Monitoring Study Definition Monitoring is defined as the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded, and reported in. It contains the e6 (r2) addenda, and provides an overview of the scope of, requirements to clinical trial monitoring process, as well as. This article discusses the principles and practice of monitoring and evaluation and. Monitoring Study Definition.
From humansofdata.atlan.com
What Is Monitoring and Evaluation? A Guide to the Basics Atlan Monitoring Study Definition They help the sponsor and the clinical research associate (cra) confirm the integrity of the study data as. Monitoring is defined as the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded, and reported in. Monitoring visits are an essential component of any clinical research project. Our first three principles link with. Monitoring Study Definition.
From theincrowdvlog.com
Monitoring and Evaluation Definition, Process, Objectives, Differences Monitoring Study Definition They help the sponsor and the clinical research associate (cra) confirm the integrity of the study data as. It contains the e6 (r2) addenda, and provides an overview of the scope of, requirements to clinical trial monitoring process, as well as. Monitoring ensure that a specific public health program to prevent and or stop epidemic is carried out as planned. Monitoring Study Definition.
From www.uni-konstanz.de
Internal accreditation / monitoring / quality assurance of study programmes Monitoring Study Definition Monitoring is defined as the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded, and reported in. Monitoring ensure that a specific public health program to prevent and or stop epidemic is carried out as planned so that the goal and objectives are meet. It contains the e6 (r2) addenda, and provides. Monitoring Study Definition.
From www.slideserve.com
PPT Environmental Mitigation and Monitoring PowerPoint Presentation Monitoring Study Definition Our first three principles link with parts a, b and c of the ich. Monitoring visits are an essential component of any clinical research project. They help the sponsor and the clinical research associate (cra) confirm the integrity of the study data as. Monitoring ensure that a specific public health program to prevent and or stop epidemic is carried out. Monitoring Study Definition.
From www.pinterest.com
a circular diagram with the words planning a monitoring and evaluation Monitoring Study Definition Our first three principles link with parts a, b and c of the ich. Monitoring ensure that a specific public health program to prevent and or stop epidemic is carried out as planned so that the goal and objectives are meet. Monitoring visits are an essential component of any clinical research project. They help the sponsor and the clinical research. Monitoring Study Definition.
From www.slideserve.com
PPT Key Terms for Monitoring and Evaluation PowerPoint Presentation Monitoring Study Definition It contains the e6 (r2) addenda, and provides an overview of the scope of, requirements to clinical trial monitoring process, as well as. Monitoring ensure that a specific public health program to prevent and or stop epidemic is carried out as planned so that the goal and objectives are meet. This article discusses the principles and practice of monitoring and. Monitoring Study Definition.
From www.slideserve.com
PPT Module 2 Monitoring and Evaluation Definitions PowerPoint Monitoring Study Definition It contains the e6 (r2) addenda, and provides an overview of the scope of, requirements to clinical trial monitoring process, as well as. They help the sponsor and the clinical research associate (cra) confirm the integrity of the study data as. This article discusses the principles and practice of monitoring and evaluation and emphasises that monitoring and evaluation (m. Monitoring. Monitoring Study Definition.
From www.slideteam.net
Five Step Monitoring And Evaluation Framework PPT Sample Monitoring Study Definition Our first three principles link with parts a, b and c of the ich. They help the sponsor and the clinical research associate (cra) confirm the integrity of the study data as. It contains the e6 (r2) addenda, and provides an overview of the scope of, requirements to clinical trial monitoring process, as well as. Monitoring ensure that a specific. Monitoring Study Definition.