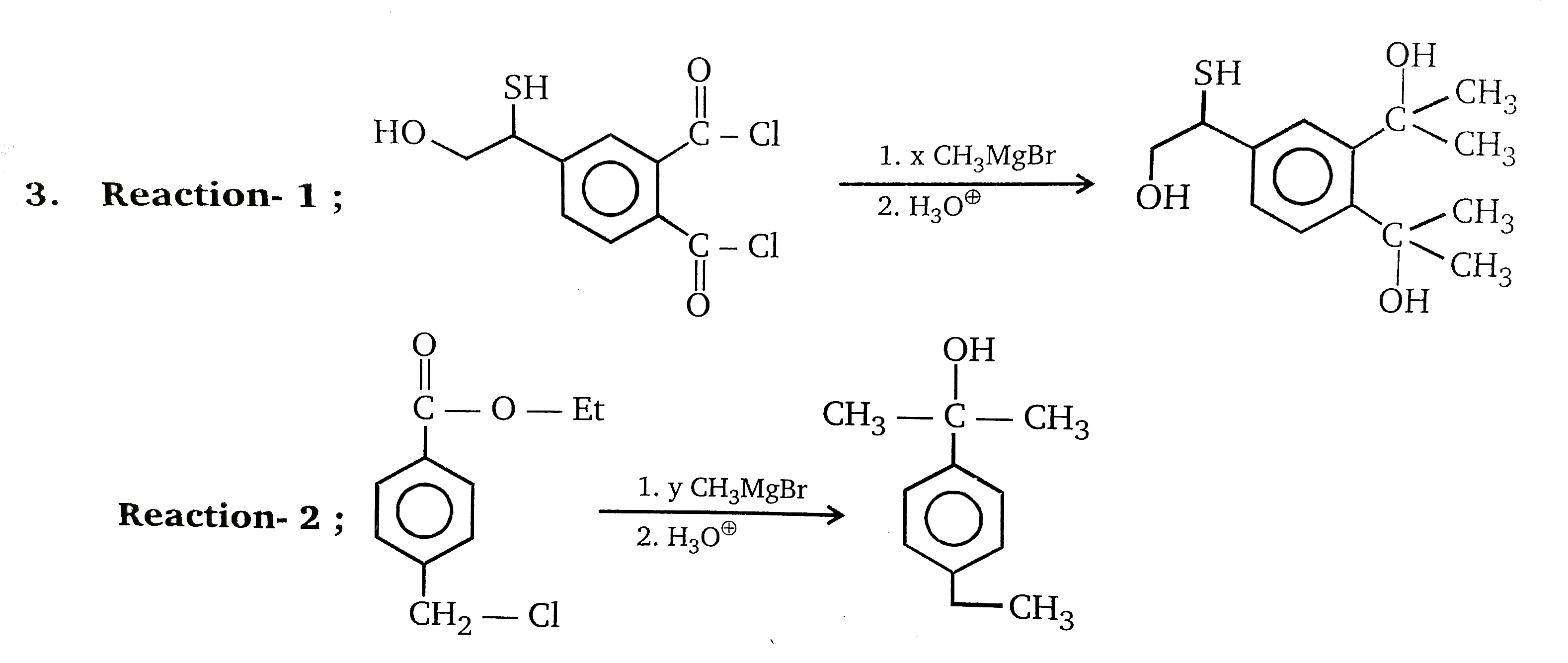
What Is The Ratio Of X/Y In Above Problem . The given product can not be obtained in the above reaction. In the given reactions, we need to determine the ratio of x/y where x and y are the equivalents of ch3mgbr used in each reaction. Y), we can ask for the ratio x/y target question: What is the value of x/y? Reaction (1) x = 6. (1) (refer to image 1) (2) (refer to image 2) in reaction 1; To find the x/y ratio in equations, isolate the ratio and simplify. 2 mole of ch3m gbr act as a base 4 mole of ch3m gbr act as a nuclophile. 2 equivalents of ch3mgbr are needed to react with 2. Asked jul 23, 2019 in chemistry by aaditrisharma ( 60.3k points) What is the ratio of (x/y) in the given problem? Use methods like solving simple equations, simultaneous equations, word. Simplify ratios or create an equivalent ratio. So for example, rather than ask for the ratio x to y (or x : The ratio calculator performs three types of operations and shows the steps to solve:
from www.doubtnut.com
Reaction (1) x = 6. Correct option is a) (1) (refer to. The ratio calculator performs three types of operations and shows the steps to solve: Y), we can ask for the ratio x/y target question: 2 mole of ch3m gbr act as a base 4 mole of ch3m gbr act as a nuclophile. What is the ratio of (x/y) in the given problem? Asked jul 23, 2019 in chemistry by aaditrisharma ( 60.3k points) In the given reactions, we need to determine the ratio of x/y where x and y are the equivalents of ch3mgbr used in each reaction. So for example, rather than ask for the ratio x to y (or x : Identify the correct product obtained.
What is the ratio of (x/y) in above problem
What Is The Ratio Of X/Y In Above Problem Identify the correct product obtained. Use methods like solving simple equations, simultaneous equations, word. Y), we can ask for the ratio x/y target question: So for example, rather than ask for the ratio x to y (or x : To find the x/y ratio in equations, isolate the ratio and simplify. Identify the correct product obtained. In the given reactions, we need to determine the ratio of x/y where x and y are the equivalents of ch3mgbr used in each reaction. Reaction (1) x = 6. (1) (refer to image 1) (2) (refer to image 2) in reaction 1; What is the value of x/y? Simplify ratios or create an equivalent ratio. Asked jul 23, 2019 in chemistry by aaditrisharma ( 60.3k points) The ratio calculator performs three types of operations and shows the steps to solve: 2 mole of ch3m gbr act as a base 4 mole of ch3m gbr act as a nuclophile. Correct option is a) (1) (refer to. What is the ratio of (x/y) in the given problem?
From brainly.com
What relationship do the ratios of Sin X and Cos y share ? What Is The Ratio Of X/Y In Above Problem Identify the correct product obtained. What is the value of x/y? Reaction (1) x = 6. 2 mole of ch3m gbr act as a base 4 mole of ch3m gbr act as a nuclophile. Use methods like solving simple equations, simultaneous equations, word. (1) (refer to image 1) (2) (refer to image 2) in reaction 1; Y), we can ask. What Is The Ratio Of X/Y In Above Problem.
From sciencenotes.org
Ratio and Proportion in Math What Is The Ratio Of X/Y In Above Problem Y), we can ask for the ratio x/y target question: Asked jul 23, 2019 in chemistry by aaditrisharma ( 60.3k points) So for example, rather than ask for the ratio x to y (or x : Correct option is a) (1) (refer to. What is the value of x/y? 2 equivalents of ch3mgbr are needed to react with 2. Use. What Is The Ratio Of X/Y In Above Problem.
From www.gauthmath.com
Pr Activity 4 Solve Me! Solve the given problem. Show your solution 1 What Is The Ratio Of X/Y In Above Problem The given product can not be obtained in the above reaction. 2 equivalents of ch3mgbr are needed to react with 2. Y), we can ask for the ratio x/y target question: Identify the correct product obtained. Reaction (1) x = 6. Asked jul 23, 2019 in chemistry by aaditrisharma ( 60.3k points) 2 mole of ch3m gbr act as a. What Is The Ratio Of X/Y In Above Problem.
From brainly.in
Let x, y and z are three positive numbers and P = x+y+z ÷2 ; if (px What Is The Ratio Of X/Y In Above Problem What is the value of x/y? Use methods like solving simple equations, simultaneous equations, word. The ratio calculator performs three types of operations and shows the steps to solve: The given product can not be obtained in the above reaction. Simplify ratios or create an equivalent ratio. So for example, rather than ask for the ratio x to y (or. What Is The Ratio Of X/Y In Above Problem.
From www.quora.com
What is the ratio of x to y in the equation 5x=7? Quora What Is The Ratio Of X/Y In Above Problem 2 mole of ch3m gbr act as a base 4 mole of ch3m gbr act as a nuclophile. Identify the correct product obtained. Use methods like solving simple equations, simultaneous equations, word. To find the x/y ratio in equations, isolate the ratio and simplify. (1) (refer to image 1) (2) (refer to image 2) in reaction 1; Reaction (1) x. What Is The Ratio Of X/Y In Above Problem.
From brainly.in
if 5x=3y=2z, what is the ratio of xyz Brainly.in What Is The Ratio Of X/Y In Above Problem (1) (refer to image 1) (2) (refer to image 2) in reaction 1; Use methods like solving simple equations, simultaneous equations, word. Correct option is a) (1) (refer to. The given product can not be obtained in the above reaction. In the given reactions, we need to determine the ratio of x/y where x and y are the equivalents of. What Is The Ratio Of X/Y In Above Problem.
From brainly.in
X square + Y square upon x squared minus y square is equal to 17 upon 8 What Is The Ratio Of X/Y In Above Problem Y), we can ask for the ratio x/y target question: What is the ratio of (x/y) in the given problem? Identify the correct product obtained. What is the value of x/y? The ratio calculator performs three types of operations and shows the steps to solve: The given product can not be obtained in the above reaction. To find the x/y. What Is The Ratio Of X/Y In Above Problem.
From brainly.com
the value of y is 20 more than the value of x. the ratio of xy = 5 What Is The Ratio Of X/Y In Above Problem Use methods like solving simple equations, simultaneous equations, word. Reaction (1) x = 6. What is the ratio of (x/y) in the given problem? The ratio calculator performs three types of operations and shows the steps to solve: Asked jul 23, 2019 in chemistry by aaditrisharma ( 60.3k points) 2 mole of ch3m gbr act as a base 4 mole. What Is The Ratio Of X/Y In Above Problem.
From www.gauthmath.com
Solved 1) The ratio of xy is 34 a) Circle the correct statement (1 What Is The Ratio Of X/Y In Above Problem So for example, rather than ask for the ratio x to y (or x : 2 mole of ch3m gbr act as a base 4 mole of ch3m gbr act as a nuclophile. Reaction (1) x = 6. What is the ratio of (x/y) in the given problem? To find the x/y ratio in equations, isolate the ratio and simplify.. What Is The Ratio Of X/Y In Above Problem.
From www.gauthmath.com
Solved Find the ratio of x to y. x/5 = 2/3 = 5/y 2/3 4/9 1 [Math] What Is The Ratio Of X/Y In Above Problem What is the value of x/y? The ratio calculator performs three types of operations and shows the steps to solve: What is the ratio of (x/y) in the given problem? Identify the correct product obtained. The given product can not be obtained in the above reaction. Reaction (1) x = 6. So for example, rather than ask for the ratio. What Is The Ratio Of X/Y In Above Problem.
From brainly.in
The ratio of x to y is 7 to 18. if the value of x is 49, then what is What Is The Ratio Of X/Y In Above Problem Correct option is a) (1) (refer to. The ratio calculator performs three types of operations and shows the steps to solve: Use methods like solving simple equations, simultaneous equations, word. Asked jul 23, 2019 in chemistry by aaditrisharma ( 60.3k points) In the given reactions, we need to determine the ratio of x/y where x and y are the equivalents. What Is The Ratio Of X/Y In Above Problem.
From www.youtube.com
How to determine the ratio of x to y in the given equations YouTube What Is The Ratio Of X/Y In Above Problem What is the value of x/y? Reaction (1) x = 6. To find the x/y ratio in equations, isolate the ratio and simplify. The ratio calculator performs three types of operations and shows the steps to solve: 2 mole of ch3m gbr act as a base 4 mole of ch3m gbr act as a nuclophile. The given product can not. What Is The Ratio Of X/Y In Above Problem.
From brainly.in
If x y = 45 and x y = 11, what is the ratio of xy? 0 1728 o 23 26 What Is The Ratio Of X/Y In Above Problem Use methods like solving simple equations, simultaneous equations, word. 2 equivalents of ch3mgbr are needed to react with 2. So for example, rather than ask for the ratio x to y (or x : The given product can not be obtained in the above reaction. 2 mole of ch3m gbr act as a base 4 mole of ch3m gbr act. What Is The Ratio Of X/Y In Above Problem.
From www.slideserve.com
PPT The ratio of X Y Z is 3 7 12. If Y is 36.4 shorter than Z What Is The Ratio Of X/Y In Above Problem Correct option is a) (1) (refer to. Reaction (1) x = 6. The given product can not be obtained in the above reaction. Identify the correct product obtained. In the given reactions, we need to determine the ratio of x/y where x and y are the equivalents of ch3mgbr used in each reaction. Asked jul 23, 2019 in chemistry by. What Is The Ratio Of X/Y In Above Problem.
From brainly.com
Find the ratio of x to y What Is The Ratio Of X/Y In Above Problem So for example, rather than ask for the ratio x to y (or x : The given product can not be obtained in the above reaction. Asked jul 23, 2019 in chemistry by aaditrisharma ( 60.3k points) Simplify ratios or create an equivalent ratio. Y), we can ask for the ratio x/y target question: The ratio calculator performs three types. What Is The Ratio Of X/Y In Above Problem.
From www.youtube.com
Writing a Ratio as an Equation Corbettmaths YouTube What Is The Ratio Of X/Y In Above Problem So for example, rather than ask for the ratio x to y (or x : (1) (refer to image 1) (2) (refer to image 2) in reaction 1; Asked jul 23, 2019 in chemistry by aaditrisharma ( 60.3k points) 2 equivalents of ch3mgbr are needed to react with 2. Correct option is a) (1) (refer to. 2 mole of ch3m. What Is The Ratio Of X/Y In Above Problem.
From www.toppr.com
X, Y and Z are partners sharing profits and losses in the ratio of 53 What Is The Ratio Of X/Y In Above Problem 2 equivalents of ch3mgbr are needed to react with 2. What is the value of x/y? So for example, rather than ask for the ratio x to y (or x : Use methods like solving simple equations, simultaneous equations, word. In the given reactions, we need to determine the ratio of x/y where x and y are the equivalents of. What Is The Ratio Of X/Y In Above Problem.
From brainly.com
Find the ratio of x to y. X/5=2/3=5/y 2/3 4/9 1 What Is The Ratio Of X/Y In Above Problem In the given reactions, we need to determine the ratio of x/y where x and y are the equivalents of ch3mgbr used in each reaction. The given product can not be obtained in the above reaction. Simplify ratios or create an equivalent ratio. Use methods like solving simple equations, simultaneous equations, word. 2 mole of ch3m gbr act as a. What Is The Ratio Of X/Y In Above Problem.
From www.teachoo.com
Example 9 Find ratio in which y−axis divides (5, 6) Examples What Is The Ratio Of X/Y In Above Problem 2 mole of ch3m gbr act as a base 4 mole of ch3m gbr act as a nuclophile. Correct option is a) (1) (refer to. Simplify ratios or create an equivalent ratio. Y), we can ask for the ratio x/y target question: (1) (refer to image 1) (2) (refer to image 2) in reaction 1; Use methods like solving simple. What Is The Ratio Of X/Y In Above Problem.
From www.teachoo.com
Example 9 Find ratio in which y−axis divides (5, 6) Examples What Is The Ratio Of X/Y In Above Problem Asked jul 23, 2019 in chemistry by aaditrisharma ( 60.3k points) The given product can not be obtained in the above reaction. Simplify ratios or create an equivalent ratio. Identify the correct product obtained. (1) (refer to image 1) (2) (refer to image 2) in reaction 1; Use methods like solving simple equations, simultaneous equations, word. 2 mole of ch3m. What Is The Ratio Of X/Y In Above Problem.
From www.toppr.com
The ratio in which Y axis divides the line joining the points ( 2,5 What Is The Ratio Of X/Y In Above Problem To find the x/y ratio in equations, isolate the ratio and simplify. In the given reactions, we need to determine the ratio of x/y where x and y are the equivalents of ch3mgbr used in each reaction. The ratio calculator performs three types of operations and shows the steps to solve: Reaction (1) x = 6. Correct option is a). What Is The Ratio Of X/Y In Above Problem.
From www.toppr.com
CH3 SH 1. x CH, MgBr 2. Hz0º OH 3. Reaction1; OH CH3 C CH3 COEt 1 What Is The Ratio Of X/Y In Above Problem In the given reactions, we need to determine the ratio of x/y where x and y are the equivalents of ch3mgbr used in each reaction. Reaction (1) x = 6. Identify the correct product obtained. 2 mole of ch3m gbr act as a base 4 mole of ch3m gbr act as a nuclophile. Use methods like solving simple equations, simultaneous. What Is The Ratio Of X/Y In Above Problem.
From brainly.in
Given that 2x/5=3y/8 find the ratio of xyI am still confused about What Is The Ratio Of X/Y In Above Problem What is the ratio of (x/y) in the given problem? Correct option is a) (1) (refer to. Identify the correct product obtained. To find the x/y ratio in equations, isolate the ratio and simplify. Reaction (1) x = 6. So for example, rather than ask for the ratio x to y (or x : Asked jul 23, 2019 in chemistry. What Is The Ratio Of X/Y In Above Problem.
From www.onlinemathlearning.com
Value of a Ratio (solutions, examples, worksheets, videos, lesson plans) What Is The Ratio Of X/Y In Above Problem 2 mole of ch3m gbr act as a base 4 mole of ch3m gbr act as a nuclophile. What is the value of x/y? Y), we can ask for the ratio x/y target question: The given product can not be obtained in the above reaction. (1) (refer to image 1) (2) (refer to image 2) in reaction 1; Correct option. What Is The Ratio Of X/Y In Above Problem.
From gmatclub.com
What is the ratio of xyz? Data Sufficiency (DS) What Is The Ratio Of X/Y In Above Problem Reaction (1) x = 6. So for example, rather than ask for the ratio x to y (or x : Asked jul 23, 2019 in chemistry by aaditrisharma ( 60.3k points) Y), we can ask for the ratio x/y target question: To find the x/y ratio in equations, isolate the ratio and simplify. 2 mole of ch3m gbr act as. What Is The Ratio Of X/Y In Above Problem.
From brainly.in
find the compound ratio of x+y x _y; x³_y³ x²_y² Brainly.in What Is The Ratio Of X/Y In Above Problem Correct option is a) (1) (refer to. Asked jul 23, 2019 in chemistry by aaditrisharma ( 60.3k points) 2 equivalents of ch3mgbr are needed to react with 2. Simplify ratios or create an equivalent ratio. In the given reactions, we need to determine the ratio of x/y where x and y are the equivalents of ch3mgbr used in each reaction.. What Is The Ratio Of X/Y In Above Problem.
From www.teachoo.com
Example 9 Find ratio in which y−axis divides (5, 6) Examples What Is The Ratio Of X/Y In Above Problem Reaction (1) x = 6. Asked jul 23, 2019 in chemistry by aaditrisharma ( 60.3k points) 2 equivalents of ch3mgbr are needed to react with 2. The given product can not be obtained in the above reaction. Simplify ratios or create an equivalent ratio. 2 mole of ch3m gbr act as a base 4 mole of ch3m gbr act as. What Is The Ratio Of X/Y In Above Problem.
From www.doubtnut.com
What is the ratio of (x/y) in above problem What Is The Ratio Of X/Y In Above Problem What is the value of x/y? (1) (refer to image 1) (2) (refer to image 2) in reaction 1; Correct option is a) (1) (refer to. What is the ratio of (x/y) in the given problem? The ratio calculator performs three types of operations and shows the steps to solve: 2 mole of ch3m gbr act as a base 4. What Is The Ratio Of X/Y In Above Problem.
From www.quora.com
What do the letters in a(Xh) ^2 +k all mean? Quora What Is The Ratio Of X/Y In Above Problem Use methods like solving simple equations, simultaneous equations, word. Y), we can ask for the ratio x/y target question: The given product can not be obtained in the above reaction. The ratio calculator performs three types of operations and shows the steps to solve: What is the value of x/y? 2 equivalents of ch3mgbr are needed to react with 2.. What Is The Ratio Of X/Y In Above Problem.
From www.doubtnut.com
What is the ratio of (x/y) in above problem What Is The Ratio Of X/Y In Above Problem To find the x/y ratio in equations, isolate the ratio and simplify. What is the ratio of (x/y) in the given problem? Simplify ratios or create an equivalent ratio. Y), we can ask for the ratio x/y target question: (1) (refer to image 1) (2) (refer to image 2) in reaction 1; Identify the correct product obtained. Correct option is. What Is The Ratio Of X/Y In Above Problem.
From brainly.in
In the given fig l//m// n. From the figure find the ratio of (x+y) (y What Is The Ratio Of X/Y In Above Problem So for example, rather than ask for the ratio x to y (or x : 2 mole of ch3m gbr act as a base 4 mole of ch3m gbr act as a nuclophile. The given product can not be obtained in the above reaction. Use methods like solving simple equations, simultaneous equations, word. In the given reactions, we need to. What Is The Ratio Of X/Y In Above Problem.
From thirdspacelearning.com
Ratio To Fraction GCSE Maths Steps, Examples & Worksheet What Is The Ratio Of X/Y In Above Problem What is the value of x/y? 2 equivalents of ch3mgbr are needed to react with 2. Y), we can ask for the ratio x/y target question: Correct option is a) (1) (refer to. To find the x/y ratio in equations, isolate the ratio and simplify. Simplify ratios or create an equivalent ratio. (1) (refer to image 1) (2) (refer to. What Is The Ratio Of X/Y In Above Problem.
From www.gauthmath.com
Solved Grid 1. What is the value of x and y if the ratio of xy is 45 What Is The Ratio Of X/Y In Above Problem To find the x/y ratio in equations, isolate the ratio and simplify. Simplify ratios or create an equivalent ratio. Use methods like solving simple equations, simultaneous equations, word. The ratio calculator performs three types of operations and shows the steps to solve: Correct option is a) (1) (refer to. What is the value of x/y? Y), we can ask for. What Is The Ratio Of X/Y In Above Problem.
From brainly.in
/ If the ratio of xy is 13 14 and x+y=81 then find the value of y What Is The Ratio Of X/Y In Above Problem To find the x/y ratio in equations, isolate the ratio and simplify. What is the value of x/y? Use methods like solving simple equations, simultaneous equations, word. In the given reactions, we need to determine the ratio of x/y where x and y are the equivalents of ch3mgbr used in each reaction. Identify the correct product obtained. 2 equivalents of. What Is The Ratio Of X/Y In Above Problem.
From www.showme.com
Extended ratio Math, Algebra, solvingequations, Proportions ShowMe What Is The Ratio Of X/Y In Above Problem Y), we can ask for the ratio x/y target question: 2 mole of ch3m gbr act as a base 4 mole of ch3m gbr act as a nuclophile. The given product can not be obtained in the above reaction. Identify the correct product obtained. What is the value of x/y? Use methods like solving simple equations, simultaneous equations, word. Simplify. What Is The Ratio Of X/Y In Above Problem.