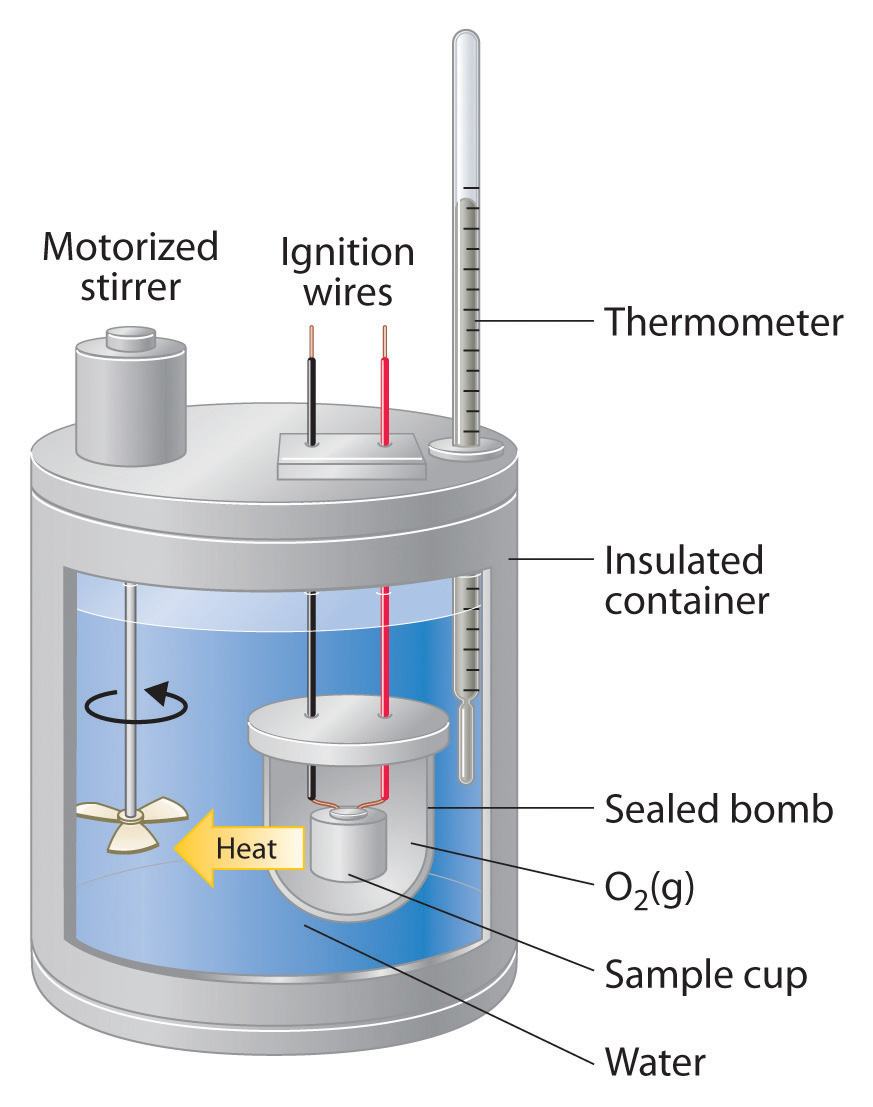
Bomb Calorimeter Is Used To Determine Mcq . In this calorimeter, the fuel is burnt at. This set of engineering chemistry multiple choice questions & answers (mcqs) focuses on “determination of calorific value of solid and. In an experiment to determine dryness fraction of steam, the mass of water separated was 1.2 kg in 15 mts and the mass of steam passed out in. Burn it and measure how much heat it releases. In bomb calorimeter, we can find the calorific value of both solid and gas fuels. A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. The bomb calorimeter is an instrument used to measure the heat of reaction at a fixed volume and the measured heat which is called the change of internal. In boy’s calorimeter we can fin calorific value for. How do you think we can determine how much energy is stored in a chemical compound? It has to withstand a large amount of pressure, in. A bomb calorimeter is used to measure the heat of combustion of a reaction.
from saylordotorg.github.io
Burn it and measure how much heat it releases. The bomb calorimeter is an instrument used to measure the heat of reaction at a fixed volume and the measured heat which is called the change of internal. In an experiment to determine dryness fraction of steam, the mass of water separated was 1.2 kg in 15 mts and the mass of steam passed out in. This set of engineering chemistry multiple choice questions & answers (mcqs) focuses on “determination of calorific value of solid and. In bomb calorimeter, we can find the calorific value of both solid and gas fuels. In this calorimeter, the fuel is burnt at. In boy’s calorimeter we can fin calorific value for. How do you think we can determine how much energy is stored in a chemical compound? It has to withstand a large amount of pressure, in. A bomb calorimeter is used to measure the heat of combustion of a reaction.
Calorimetry
Bomb Calorimeter Is Used To Determine Mcq It has to withstand a large amount of pressure, in. In bomb calorimeter, we can find the calorific value of both solid and gas fuels. In an experiment to determine dryness fraction of steam, the mass of water separated was 1.2 kg in 15 mts and the mass of steam passed out in. The bomb calorimeter is an instrument used to measure the heat of reaction at a fixed volume and the measured heat which is called the change of internal. A bomb calorimeter is used to measure the heat of combustion of a reaction. In this calorimeter, the fuel is burnt at. This set of engineering chemistry multiple choice questions & answers (mcqs) focuses on “determination of calorific value of solid and. A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. How do you think we can determine how much energy is stored in a chemical compound? In boy’s calorimeter we can fin calorific value for. It has to withstand a large amount of pressure, in. Burn it and measure how much heat it releases.
From www.iitianacademy.com
AP Chemistry 6.4 Heat Capacity and Calorimetry Exam Style questions Bomb Calorimeter Is Used To Determine Mcq A bomb calorimeter is used to measure the heat of combustion of a reaction. It has to withstand a large amount of pressure, in. Burn it and measure how much heat it releases. In boy’s calorimeter we can fin calorific value for. In an experiment to determine dryness fraction of steam, the mass of water separated was 1.2 kg in. Bomb Calorimeter Is Used To Determine Mcq.
From biopchem.education
Parr ‘Bomb’ Calorimeter BioPchem Bomb Calorimeter Is Used To Determine Mcq In boy’s calorimeter we can fin calorific value for. A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. In this calorimeter, the fuel is burnt at. How do you think we can determine how much energy is stored in a chemical compound? In an experiment to determine dryness fraction of steam, the mass. Bomb Calorimeter Is Used To Determine Mcq.
From www.slideserve.com
PPT Chapter 6 Thermochemistry PowerPoint Presentation, free download Bomb Calorimeter Is Used To Determine Mcq It has to withstand a large amount of pressure, in. This set of engineering chemistry multiple choice questions & answers (mcqs) focuses on “determination of calorific value of solid and. In this calorimeter, the fuel is burnt at. In boy’s calorimeter we can fin calorific value for. In bomb calorimeter, we can find the calorific value of both solid and. Bomb Calorimeter Is Used To Determine Mcq.
From www.numerade.com
SOLVED Use the References to access important values if needed for Bomb Calorimeter Is Used To Determine Mcq A bomb calorimeter is used to measure the heat of combustion of a reaction. It has to withstand a large amount of pressure, in. In an experiment to determine dryness fraction of steam, the mass of water separated was 1.2 kg in 15 mts and the mass of steam passed out in. How do you think we can determine how. Bomb Calorimeter Is Used To Determine Mcq.
From www.pinterest.com
ConstantVolume Calorimetry for more precise work than the coffeecup Bomb Calorimeter Is Used To Determine Mcq The bomb calorimeter is an instrument used to measure the heat of reaction at a fixed volume and the measured heat which is called the change of internal. How do you think we can determine how much energy is stored in a chemical compound? In boy’s calorimeter we can fin calorific value for. A bomb calorimeter is used for finding. Bomb Calorimeter Is Used To Determine Mcq.
From engineeringlearn.com
Bomb Calorimeter Definition, Construction, Diagram, Working & Uses Bomb Calorimeter Is Used To Determine Mcq Burn it and measure how much heat it releases. In an experiment to determine dryness fraction of steam, the mass of water separated was 1.2 kg in 15 mts and the mass of steam passed out in. In bomb calorimeter, we can find the calorific value of both solid and gas fuels. The bomb calorimeter is an instrument used to. Bomb Calorimeter Is Used To Determine Mcq.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimeter Is Used To Determine Mcq How do you think we can determine how much energy is stored in a chemical compound? In bomb calorimeter, we can find the calorific value of both solid and gas fuels. In boy’s calorimeter we can fin calorific value for. Burn it and measure how much heat it releases. This set of engineering chemistry multiple choice questions & answers (mcqs). Bomb Calorimeter Is Used To Determine Mcq.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Bomb Calorimeter Is Used To Determine Mcq How do you think we can determine how much energy is stored in a chemical compound? In boy’s calorimeter we can fin calorific value for. Burn it and measure how much heat it releases. In bomb calorimeter, we can find the calorific value of both solid and gas fuels. It has to withstand a large amount of pressure, in. In. Bomb Calorimeter Is Used To Determine Mcq.
From www.bartleby.com
Answered A bomb calorimeter, or a constant… bartleby Bomb Calorimeter Is Used To Determine Mcq In an experiment to determine dryness fraction of steam, the mass of water separated was 1.2 kg in 15 mts and the mass of steam passed out in. In bomb calorimeter, we can find the calorific value of both solid and gas fuels. Burn it and measure how much heat it releases. How do you think we can determine how. Bomb Calorimeter Is Used To Determine Mcq.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 3.1.2 Calorimetry翰林国际教育 Bomb Calorimeter Is Used To Determine Mcq Burn it and measure how much heat it releases. In boy’s calorimeter we can fin calorific value for. How do you think we can determine how much energy is stored in a chemical compound? It has to withstand a large amount of pressure, in. A bomb calorimeter is used to measure the heat of combustion of a reaction. In bomb. Bomb Calorimeter Is Used To Determine Mcq.
From www.athenatechnology.co.in
Athena Technology Bomb Calorimeter Is Used To Determine Mcq How do you think we can determine how much energy is stored in a chemical compound? In bomb calorimeter, we can find the calorific value of both solid and gas fuels. In an experiment to determine dryness fraction of steam, the mass of water separated was 1.2 kg in 15 mts and the mass of steam passed out in. The. Bomb Calorimeter Is Used To Determine Mcq.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Bomb Calorimeter Is Used To Determine Mcq In this calorimeter, the fuel is burnt at. The bomb calorimeter is an instrument used to measure the heat of reaction at a fixed volume and the measured heat which is called the change of internal. A bomb calorimeter is used to measure the heat of combustion of a reaction. How do you think we can determine how much energy. Bomb Calorimeter Is Used To Determine Mcq.
From wingle.jp
😂 Bomb calorimeter experiment conclusion. Experiment 1 A3 Bomb Bomb Calorimeter Is Used To Determine Mcq In an experiment to determine dryness fraction of steam, the mass of water separated was 1.2 kg in 15 mts and the mass of steam passed out in. A bomb calorimeter is used to measure the heat of combustion of a reaction. How do you think we can determine how much energy is stored in a chemical compound? In bomb. Bomb Calorimeter Is Used To Determine Mcq.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Bomb Calorimeter Is Used To Determine Mcq In this calorimeter, the fuel is burnt at. How do you think we can determine how much energy is stored in a chemical compound? A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. In an experiment to determine dryness fraction of steam, the mass of water separated was 1.2 kg in 15 mts. Bomb Calorimeter Is Used To Determine Mcq.
From ar.inspiredpencil.com
Simple Bomb Calorimeter Bomb Calorimeter Is Used To Determine Mcq Burn it and measure how much heat it releases. This set of engineering chemistry multiple choice questions & answers (mcqs) focuses on “determination of calorific value of solid and. A bomb calorimeter is used to measure the heat of combustion of a reaction. In this calorimeter, the fuel is burnt at. A bomb calorimeter is used for finding the higher. Bomb Calorimeter Is Used To Determine Mcq.
From users.highland.edu
Calorimetry Bomb Calorimeter Is Used To Determine Mcq This set of engineering chemistry multiple choice questions & answers (mcqs) focuses on “determination of calorific value of solid and. In an experiment to determine dryness fraction of steam, the mass of water separated was 1.2 kg in 15 mts and the mass of steam passed out in. A bomb calorimeter is used to measure the heat of combustion of. Bomb Calorimeter Is Used To Determine Mcq.
From ar.inspiredpencil.com
Bomb Calorimeter Setup Bomb Calorimeter Is Used To Determine Mcq The bomb calorimeter is an instrument used to measure the heat of reaction at a fixed volume and the measured heat which is called the change of internal. It has to withstand a large amount of pressure, in. In an experiment to determine dryness fraction of steam, the mass of water separated was 1.2 kg in 15 mts and the. Bomb Calorimeter Is Used To Determine Mcq.
From exoqfqtuz.blob.core.windows.net
Calorimetry Class 11 Questions at Vivian Higgins blog Bomb Calorimeter Is Used To Determine Mcq The bomb calorimeter is an instrument used to measure the heat of reaction at a fixed volume and the measured heat which is called the change of internal. In boy’s calorimeter we can fin calorific value for. In this calorimeter, the fuel is burnt at. In an experiment to determine dryness fraction of steam, the mass of water separated was. Bomb Calorimeter Is Used To Determine Mcq.
From martinfersbanks.blogspot.com
Is a Bomb Calorimeter Constant Pressure Bomb Calorimeter Is Used To Determine Mcq The bomb calorimeter is an instrument used to measure the heat of reaction at a fixed volume and the measured heat which is called the change of internal. This set of engineering chemistry multiple choice questions & answers (mcqs) focuses on “determination of calorific value of solid and. In this calorimeter, the fuel is burnt at. How do you think. Bomb Calorimeter Is Used To Determine Mcq.
From www.chegg.com
Solved A bomb calorimeter, or constant volume calorimeter, Bomb Calorimeter Is Used To Determine Mcq In this calorimeter, the fuel is burnt at. Burn it and measure how much heat it releases. In bomb calorimeter, we can find the calorific value of both solid and gas fuels. In an experiment to determine dryness fraction of steam, the mass of water separated was 1.2 kg in 15 mts and the mass of steam passed out in.. Bomb Calorimeter Is Used To Determine Mcq.
From www.labxyi.com
CBC900 Compact automatic oxygen bomb calorimeter Bomb Calorimeter Is Used To Determine Mcq Burn it and measure how much heat it releases. This set of engineering chemistry multiple choice questions & answers (mcqs) focuses on “determination of calorific value of solid and. In an experiment to determine dryness fraction of steam, the mass of water separated was 1.2 kg in 15 mts and the mass of steam passed out in. In boy’s calorimeter. Bomb Calorimeter Is Used To Determine Mcq.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Bomb Calorimeter Is Used To Determine Mcq A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. Burn it and measure how much heat it releases. In bomb calorimeter, we can find the calorific value of both solid and gas fuels. In this calorimeter, the fuel is burnt at. This set of engineering chemistry multiple choice questions & answers (mcqs) focuses. Bomb Calorimeter Is Used To Determine Mcq.
From www.bartleby.com
Answered In the laboratory a "coffee cup"… bartleby Bomb Calorimeter Is Used To Determine Mcq How do you think we can determine how much energy is stored in a chemical compound? In boy’s calorimeter we can fin calorific value for. Burn it and measure how much heat it releases. In bomb calorimeter, we can find the calorific value of both solid and gas fuels. It has to withstand a large amount of pressure, in. A. Bomb Calorimeter Is Used To Determine Mcq.
From www.pinterest.com
Calorimetry Bomb Calorimeter Experiment Science fair, Homeschool and Bomb Calorimeter Is Used To Determine Mcq A bomb calorimeter is used to measure the heat of combustion of a reaction. In boy’s calorimeter we can fin calorific value for. It has to withstand a large amount of pressure, in. A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. Burn it and measure how much heat it releases. In an. Bomb Calorimeter Is Used To Determine Mcq.
From saylordotorg.github.io
Calorimetry Bomb Calorimeter Is Used To Determine Mcq Burn it and measure how much heat it releases. How do you think we can determine how much energy is stored in a chemical compound? A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. A bomb calorimeter is used to measure the heat of combustion of a reaction. In bomb calorimeter, we can. Bomb Calorimeter Is Used To Determine Mcq.
From www.studocu.com
Bomb Calorimeter Problems Bomb Calorimeter Practice Problems Name Bomb Calorimeter Is Used To Determine Mcq In bomb calorimeter, we can find the calorific value of both solid and gas fuels. A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. This set of engineering chemistry multiple choice questions & answers (mcqs) focuses on “determination of calorific value of solid and. In this calorimeter, the fuel is burnt at. A. Bomb Calorimeter Is Used To Determine Mcq.
From www.numerade.com
SOLVED A bomb calorimeter or a constant volume calorimeler; is a Bomb Calorimeter Is Used To Determine Mcq In an experiment to determine dryness fraction of steam, the mass of water separated was 1.2 kg in 15 mts and the mass of steam passed out in. This set of engineering chemistry multiple choice questions & answers (mcqs) focuses on “determination of calorific value of solid and. A bomb calorimeter is used to measure the heat of combustion of. Bomb Calorimeter Is Used To Determine Mcq.
From animalia-life.club
Simple Bomb Calorimeter Bomb Calorimeter Is Used To Determine Mcq A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. In boy’s calorimeter we can fin calorific value for. How do you think we can determine how much energy is stored in a chemical compound? The bomb calorimeter is an instrument used to measure the heat of reaction at a fixed volume and the. Bomb Calorimeter Is Used To Determine Mcq.
From www.numerade.com
SOLVED To calculate the enthalpy of combustion using bomb calorimeter Bomb Calorimeter Is Used To Determine Mcq How do you think we can determine how much energy is stored in a chemical compound? In this calorimeter, the fuel is burnt at. A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. The bomb calorimeter is an instrument used to measure the heat of reaction at a fixed volume and the measured. Bomb Calorimeter Is Used To Determine Mcq.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Bomb Calorimeter Is Used To Determine Mcq In this calorimeter, the fuel is burnt at. Burn it and measure how much heat it releases. In boy’s calorimeter we can fin calorific value for. A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. In bomb calorimeter, we can find the calorific value of both solid and gas fuels. In an experiment. Bomb Calorimeter Is Used To Determine Mcq.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Bomb Calorimeter Is Used To Determine Mcq A bomb calorimeter is used to measure the heat of combustion of a reaction. How do you think we can determine how much energy is stored in a chemical compound? In boy’s calorimeter we can fin calorific value for. The bomb calorimeter is an instrument used to measure the heat of reaction at a fixed volume and the measured heat. Bomb Calorimeter Is Used To Determine Mcq.
From schoolbag.info
Figure 7.6. Diagram of a Bomb Calorimeter Bomb Calorimeter Is Used To Determine Mcq In bomb calorimeter, we can find the calorific value of both solid and gas fuels. The bomb calorimeter is an instrument used to measure the heat of reaction at a fixed volume and the measured heat which is called the change of internal. In an experiment to determine dryness fraction of steam, the mass of water separated was 1.2 kg. Bomb Calorimeter Is Used To Determine Mcq.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Bomb Calorimeter Is Used To Determine Mcq A bomb calorimeter is used for finding the higher calorific value of solid and liquid fuels. It has to withstand a large amount of pressure, in. The bomb calorimeter is an instrument used to measure the heat of reaction at a fixed volume and the measured heat which is called the change of internal. This set of engineering chemistry multiple. Bomb Calorimeter Is Used To Determine Mcq.
From www.biophlox.com
Buy Bomb Calorimeter get price for lab equipment Bomb Calorimeter Is Used To Determine Mcq Burn it and measure how much heat it releases. This set of engineering chemistry multiple choice questions & answers (mcqs) focuses on “determination of calorific value of solid and. In bomb calorimeter, we can find the calorific value of both solid and gas fuels. In boy’s calorimeter we can fin calorific value for. How do you think we can determine. Bomb Calorimeter Is Used To Determine Mcq.
From gamma.app
Bomb Calorimeter A Comprehensive Guide Bomb Calorimeter Is Used To Determine Mcq It has to withstand a large amount of pressure, in. Burn it and measure how much heat it releases. This set of engineering chemistry multiple choice questions & answers (mcqs) focuses on “determination of calorific value of solid and. A bomb calorimeter is used to measure the heat of combustion of a reaction. The bomb calorimeter is an instrument used. Bomb Calorimeter Is Used To Determine Mcq.