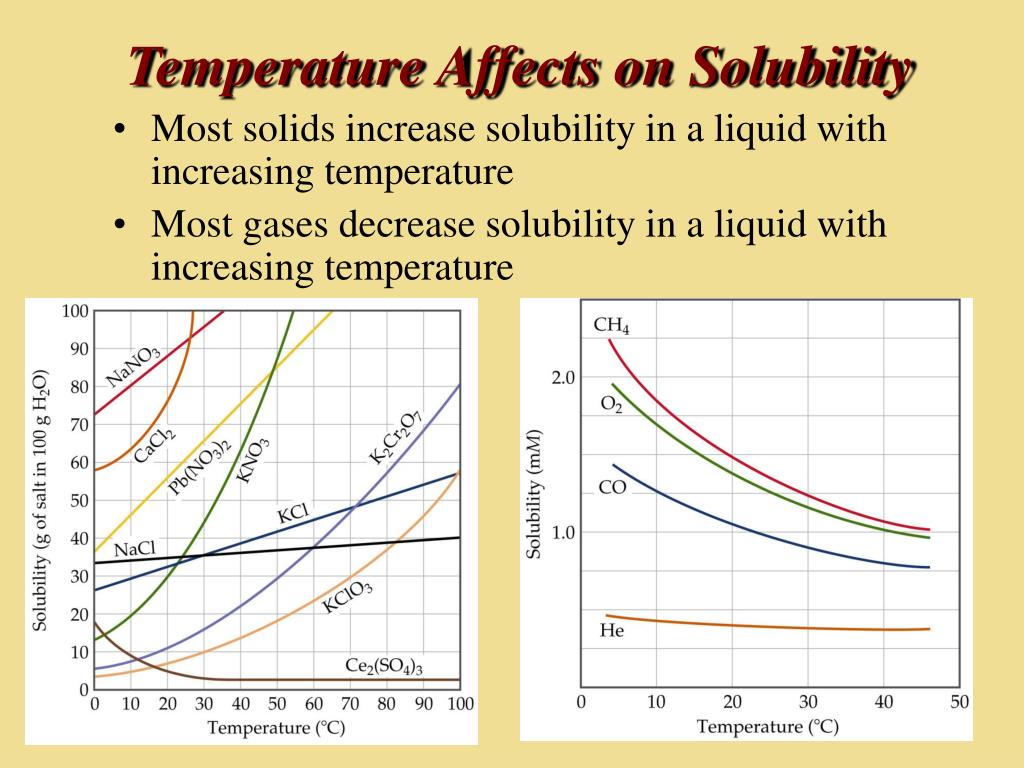
Temperature On Solubility Of Solids In Liquids . Solubility of all three solutes increases as temperature increases. The components of a mixture can often be separated using fractional crystallization ,. The solubility of most solid or liquid solutes increases with increasing temperature. Temperature changes affect the solubility of solids, liquids and gases differently. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in figure 11.16. The solubility of a solid in water increases with an increase in temperature. A solubility curve is a graph of the solubility vs. The solubility of potassium nitrate increases the most from approximately 30 g per 100 g. When a solid dissolves in a liquid, a change in the physical state of the solid. The solubility of solutes is dependent on temperature. Gas solubility decreases as the temperature.
from www.slideserve.com
The solubility of potassium nitrate increases the most from approximately 30 g per 100 g. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in figure 11.16. The solubility of a solid in water increases with an increase in temperature. Temperature changes affect the solubility of solids, liquids and gases differently. Solubility of all three solutes increases as temperature increases. When a solid dissolves in a liquid, a change in the physical state of the solid. The solubility of most solid or liquid solutes increases with increasing temperature. The solubility of solutes is dependent on temperature. A solubility curve is a graph of the solubility vs. Gas solubility decreases as the temperature.
PPT Solubility PowerPoint Presentation, free download ID37453
Temperature On Solubility Of Solids In Liquids The solubility of a solid in water increases with an increase in temperature. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in figure 11.16. The solubility of a solid in water increases with an increase in temperature. The solubility of most solid or liquid solutes increases with increasing temperature. Solubility of all three solutes increases as temperature increases. Temperature changes affect the solubility of solids, liquids and gases differently. The components of a mixture can often be separated using fractional crystallization ,. The solubility of solutes is dependent on temperature. The solubility of potassium nitrate increases the most from approximately 30 g per 100 g. A solubility curve is a graph of the solubility vs. Gas solubility decreases as the temperature. When a solid dissolves in a liquid, a change in the physical state of the solid.
From www.youtube.com
Solubility of Solids at Different Temperatures Experiment YouTube Temperature On Solubility Of Solids In Liquids The solubility of a solid in water increases with an increase in temperature. A solubility curve is a graph of the solubility vs. Gas solubility decreases as the temperature. When a solid dissolves in a liquid, a change in the physical state of the solid. The solubility of most solid or liquid solutes increases with increasing temperature. The components of. Temperature On Solubility Of Solids In Liquids.
From chem.libretexts.org
13.4 Effects of Temperature and Pressure on Solubility Chemistry Temperature On Solubility Of Solids In Liquids The solubility of solutes is dependent on temperature. Solubility of all three solutes increases as temperature increases. When a solid dissolves in a liquid, a change in the physical state of the solid. The solubility of most solid or liquid solutes increases with increasing temperature. Temperature changes affect the solubility of solids, liquids and gases differently. The solubility of a. Temperature On Solubility Of Solids In Liquids.
From ecurrencythailand.com
What Is The Effect Of Temperature On The Solubility Of A Solid In A Temperature On Solubility Of Solids In Liquids The solubility of solutes is dependent on temperature. A solubility curve is a graph of the solubility vs. Temperature changes affect the solubility of solids, liquids and gases differently. The components of a mixture can often be separated using fractional crystallization ,. The solubility of a solid in water increases with an increase in temperature. The solubility of most solid. Temperature On Solubility Of Solids In Liquids.
From www.vecteezy.com
Changing the state of matter from solid, liquid and gas due to Temperature On Solubility Of Solids In Liquids The solubility of a solid in water increases with an increase in temperature. Gas solubility decreases as the temperature. Temperature changes affect the solubility of solids, liquids and gases differently. Solubility of all three solutes increases as temperature increases. The components of a mixture can often be separated using fractional crystallization ,. The solubility of potassium nitrate increases the most. Temperature On Solubility Of Solids In Liquids.
From classnotes.org.in
Solubility of Gases and Solids in Liquids Chemistry, Class 12, Solutions Temperature On Solubility Of Solids In Liquids The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in figure 11.16. The solubility of most solid or liquid solutes increases with increasing temperature. Gas solubility decreases as the temperature. The solubility of potassium nitrate increases the most from approximately 30 g per 100 g. A solubility curve is a. Temperature On Solubility Of Solids In Liquids.
From tinnongtuyensinh.com
Exploring Temperatures Impact On Solubility Solid Vs. Gas Temperature On Solubility Of Solids In Liquids Temperature changes affect the solubility of solids, liquids and gases differently. Solubility of all three solutes increases as temperature increases. The solubility of most solid or liquid solutes increases with increasing temperature. When a solid dissolves in a liquid, a change in the physical state of the solid. The solubility of solutes is dependent on temperature. A solubility curve is. Temperature On Solubility Of Solids In Liquids.
From courses.lumenlearning.com
Gas Solubility and Temperature Introduction to Chemistry Temperature On Solubility Of Solids In Liquids The solubility of most solid or liquid solutes increases with increasing temperature. Solubility of all three solutes increases as temperature increases. The solubility of a solid in water increases with an increase in temperature. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in figure 11.16. The solubility of potassium. Temperature On Solubility Of Solids In Liquids.
From www.vedantu.com
What is the relationship between the solubility and the temperature Temperature On Solubility Of Solids In Liquids The solubility of most solid or liquid solutes increases with increasing temperature. The solubility of potassium nitrate increases the most from approximately 30 g per 100 g. The solubility of solutes is dependent on temperature. The solubility of a solid in water increases with an increase in temperature. The dependence of solubility on temperature for a number of solids in. Temperature On Solubility Of Solids In Liquids.
From www.vrogue.co
How Does Temperature Affect Solids Liquids And Gases vrogue.co Temperature On Solubility Of Solids In Liquids The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in figure 11.16. The solubility of potassium nitrate increases the most from approximately 30 g per 100 g. The components of a mixture can often be separated using fractional crystallization ,. Gas solubility decreases as the temperature. Solubility of all three. Temperature On Solubility Of Solids In Liquids.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID3105812 Temperature On Solubility Of Solids In Liquids The solubility of most solid or liquid solutes increases with increasing temperature. When a solid dissolves in a liquid, a change in the physical state of the solid. The components of a mixture can often be separated using fractional crystallization ,. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves. Temperature On Solubility Of Solids In Liquids.
From www.breakingatom.com
Solubility of Elements and Compounds Temperature On Solubility Of Solids In Liquids The components of a mixture can often be separated using fractional crystallization ,. Temperature changes affect the solubility of solids, liquids and gases differently. The solubility of most solid or liquid solutes increases with increasing temperature. A solubility curve is a graph of the solubility vs. Solubility of all three solutes increases as temperature increases. Gas solubility decreases as the. Temperature On Solubility Of Solids In Liquids.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID37453 Temperature On Solubility Of Solids In Liquids The solubility of potassium nitrate increases the most from approximately 30 g per 100 g. The components of a mixture can often be separated using fractional crystallization ,. Solubility of all three solutes increases as temperature increases. The solubility of solutes is dependent on temperature. When a solid dissolves in a liquid, a change in the physical state of the. Temperature On Solubility Of Solids In Liquids.
From www.slideserve.com
PPT Introduction to Solutions PowerPoint Presentation, free download Temperature On Solubility Of Solids In Liquids Gas solubility decreases as the temperature. Temperature changes affect the solubility of solids, liquids and gases differently. The solubility of a solid in water increases with an increase in temperature. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in figure 11.16. A solubility curve is a graph of the. Temperature On Solubility Of Solids In Liquids.
From www.blendspace.com
Chapter 14 Aqueous Solutions Lessons Blendspace Temperature On Solubility Of Solids In Liquids The components of a mixture can often be separated using fractional crystallization ,. Solubility of all three solutes increases as temperature increases. The solubility of solutes is dependent on temperature. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in figure 11.16. Temperature changes affect the solubility of solids, liquids. Temperature On Solubility Of Solids In Liquids.
From mungfali.com
Solids Liquids Gases Chart Temperature On Solubility Of Solids In Liquids The solubility of solutes is dependent on temperature. The solubility of a solid in water increases with an increase in temperature. Gas solubility decreases as the temperature. The components of a mixture can often be separated using fractional crystallization ,. Temperature changes affect the solubility of solids, liquids and gases differently. The solubility of potassium nitrate increases the most from. Temperature On Solubility Of Solids In Liquids.
From www.ck12.org
Solubility CK12 Foundation Temperature On Solubility Of Solids In Liquids Solubility of all three solutes increases as temperature increases. The solubility of a solid in water increases with an increase in temperature. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in figure 11.16. When a solid dissolves in a liquid, a change in the physical state of the solid.. Temperature On Solubility Of Solids In Liquids.
From www.slideserve.com
PPT Solutions and Mixtures PowerPoint Presentation, free download Temperature On Solubility Of Solids In Liquids Solubility of all three solutes increases as temperature increases. The solubility of solutes is dependent on temperature. The solubility of a solid in water increases with an increase in temperature. The solubility of potassium nitrate increases the most from approximately 30 g per 100 g. Gas solubility decreases as the temperature. The solubility of most solid or liquid solutes increases. Temperature On Solubility Of Solids In Liquids.
From www.slideserve.com
PPT Solubility Notes PowerPoint Presentation, free download ID5606676 Temperature On Solubility Of Solids In Liquids When a solid dissolves in a liquid, a change in the physical state of the solid. The solubility of a solid in water increases with an increase in temperature. The solubility of solutes is dependent on temperature. The solubility of potassium nitrate increases the most from approximately 30 g per 100 g. The components of a mixture can often be. Temperature On Solubility Of Solids In Liquids.
From duanvanphu.com
What Is The Effect Of Solubility On Temperature In Class 9 Science? Temperature On Solubility Of Solids In Liquids The solubility of a solid in water increases with an increase in temperature. The solubility of solutes is dependent on temperature. When a solid dissolves in a liquid, a change in the physical state of the solid. The solubility of potassium nitrate increases the most from approximately 30 g per 100 g. Gas solubility decreases as the temperature. A solubility. Temperature On Solubility Of Solids In Liquids.
From ppt-online.org
Disperse systems. True solution презентация онлайн Temperature On Solubility Of Solids In Liquids When a solid dissolves in a liquid, a change in the physical state of the solid. The solubility of most solid or liquid solutes increases with increasing temperature. The solubility of a solid in water increases with an increase in temperature. The solubility of solutes is dependent on temperature. Solubility of all three solutes increases as temperature increases. The dependence. Temperature On Solubility Of Solids In Liquids.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation Temperature On Solubility Of Solids In Liquids The solubility of most solid or liquid solutes increases with increasing temperature. Gas solubility decreases as the temperature. Solubility of all three solutes increases as temperature increases. The solubility of solutes is dependent on temperature. The solubility of potassium nitrate increases the most from approximately 30 g per 100 g. The components of a mixture can often be separated using. Temperature On Solubility Of Solids In Liquids.
From infogram.com
Solubility Infographic Infogram Temperature On Solubility Of Solids In Liquids The solubility of solutes is dependent on temperature. The components of a mixture can often be separated using fractional crystallization ,. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in figure 11.16. When a solid dissolves in a liquid, a change in the physical state of the solid. Solubility. Temperature On Solubility Of Solids In Liquids.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID5813207 Temperature On Solubility Of Solids In Liquids The solubility of potassium nitrate increases the most from approximately 30 g per 100 g. The components of a mixture can often be separated using fractional crystallization ,. When a solid dissolves in a liquid, a change in the physical state of the solid. Solubility of all three solutes increases as temperature increases. Temperature changes affect the solubility of solids,. Temperature On Solubility Of Solids In Liquids.
From solutionpharmacy.in
Solubility of Gas in Liquids Solution Parmacy Temperature On Solubility Of Solids In Liquids The solubility of most solid or liquid solutes increases with increasing temperature. The solubility of solutes is dependent on temperature. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in figure 11.16. The components of a mixture can often be separated using fractional crystallization ,. Solubility of all three solutes. Temperature On Solubility Of Solids In Liquids.
From www.docsity.com
Solubility General Chemistry Lecture Slides Docsity Temperature On Solubility Of Solids In Liquids The solubility of solutes is dependent on temperature. The solubility of a solid in water increases with an increase in temperature. The solubility of potassium nitrate increases the most from approximately 30 g per 100 g. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in figure 11.16. A solubility. Temperature On Solubility Of Solids In Liquids.
From www.numerade.com
SOLVEDHow does temperature affect the solubility of a solid in a Temperature On Solubility Of Solids In Liquids A solubility curve is a graph of the solubility vs. Solubility of all three solutes increases as temperature increases. The components of a mixture can often be separated using fractional crystallization ,. Gas solubility decreases as the temperature. The solubility of potassium nitrate increases the most from approximately 30 g per 100 g. The solubility of most solid or liquid. Temperature On Solubility Of Solids In Liquids.
From chem.libretexts.org
Solubility and Precipitation Chemistry LibreTexts Temperature On Solubility Of Solids In Liquids The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in figure 11.16. The solubility of solutes is dependent on temperature. A solubility curve is a graph of the solubility vs. Gas solubility decreases as the temperature. The components of a mixture can often be separated using fractional crystallization ,. The. Temperature On Solubility Of Solids In Liquids.
From samson-jolpblogsantos.blogspot.com
How Does Temperature Affect Solids Liquids and Gases Temperature On Solubility Of Solids In Liquids A solubility curve is a graph of the solubility vs. Gas solubility decreases as the temperature. The solubility of most solid or liquid solutes increases with increasing temperature. When a solid dissolves in a liquid, a change in the physical state of the solid. The dependence of solubility on temperature for a number of solids in water is shown by. Temperature On Solubility Of Solids In Liquids.
From www.slideserve.com
PPT Chapter 7 Solutions and Colloids PowerPoint Presentation, free Temperature On Solubility Of Solids In Liquids The solubility of potassium nitrate increases the most from approximately 30 g per 100 g. The solubility of a solid in water increases with an increase in temperature. When a solid dissolves in a liquid, a change in the physical state of the solid. A solubility curve is a graph of the solubility vs. The components of a mixture can. Temperature On Solubility Of Solids In Liquids.
From www.slideserve.com
PPT Factors Affecting Solubility PowerPoint Presentation, free Temperature On Solubility Of Solids In Liquids Temperature changes affect the solubility of solids, liquids and gases differently. Gas solubility decreases as the temperature. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in figure 11.16. Solubility of all three solutes increases as temperature increases. The solubility of most solid or liquid solutes increases with increasing temperature.. Temperature On Solubility Of Solids In Liquids.
From www.dreamstime.com
Solubility Vector Illustration. Labeled Solute, Solvent and Solution Temperature On Solubility Of Solids In Liquids Solubility of all three solutes increases as temperature increases. The solubility of potassium nitrate increases the most from approximately 30 g per 100 g. When a solid dissolves in a liquid, a change in the physical state of the solid. Temperature changes affect the solubility of solids, liquids and gases differently. The solubility of solutes is dependent on temperature. The. Temperature On Solubility Of Solids In Liquids.
From 2012books.lardbucket.org
Effects of Temperature and Pressure on Solubility Temperature On Solubility Of Solids In Liquids When a solid dissolves in a liquid, a change in the physical state of the solid. Temperature changes affect the solubility of solids, liquids and gases differently. The solubility of most solid or liquid solutes increases with increasing temperature. The components of a mixture can often be separated using fractional crystallization ,. The solubility of a solid in water increases. Temperature On Solubility Of Solids In Liquids.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID5581895 Temperature On Solubility Of Solids In Liquids The solubility of most solid or liquid solutes increases with increasing temperature. Solubility of all three solutes increases as temperature increases. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in figure 11.16. A solubility curve is a graph of the solubility vs. When a solid dissolves in a liquid,. Temperature On Solubility Of Solids In Liquids.
From www.youtube.com
Effect of temperature and pressure on solubility of solids and gases Temperature On Solubility Of Solids In Liquids When a solid dissolves in a liquid, a change in the physical state of the solid. Solubility of all three solutes increases as temperature increases. The solubility of a solid in water increases with an increase in temperature. The solubility of most solid or liquid solutes increases with increasing temperature. Gas solubility decreases as the temperature. The components of a. Temperature On Solubility Of Solids In Liquids.
From media.ed.science.psu.edu
Solubility of ionic solids as a function of temperature Temperature On Solubility Of Solids In Liquids When a solid dissolves in a liquid, a change in the physical state of the solid. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in figure 11.16. Solubility of all three solutes increases as temperature increases. The solubility of most solid or liquid solutes increases with increasing temperature. The. Temperature On Solubility Of Solids In Liquids.