What Is Medical Device Clinical Trials . A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a. In the context of medical devices, a clinical trial or a clinical investigation can be defined as any systematic investigation or study on one or more human subjects undertaken to assess. Find out more about what is required for clinical trials of medical devices. A clinical trial is a research study of a health product to investigate any of the following in humans: “an instrument…intended for use in the “diagnosis…cure,. Discover or verify its clinical,. This review describes the fda. An applicable device clinical trial is a prospective clinical study of health outcomes comparing an intervention with a device against a control in. As defined in section 201 of the federal food, drug, and cosmetic act, a medical device is:
from www.abbvieclinicaltrials.com
This review describes the fda. A clinical trial is a research study of a health product to investigate any of the following in humans: An applicable device clinical trial is a prospective clinical study of health outcomes comparing an intervention with a device against a control in. Find out more about what is required for clinical trials of medical devices. Discover or verify its clinical,. “an instrument…intended for use in the “diagnosis…cure,. As defined in section 201 of the federal food, drug, and cosmetic act, a medical device is: A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a. In the context of medical devices, a clinical trial or a clinical investigation can be defined as any systematic investigation or study on one or more human subjects undertaken to assess.
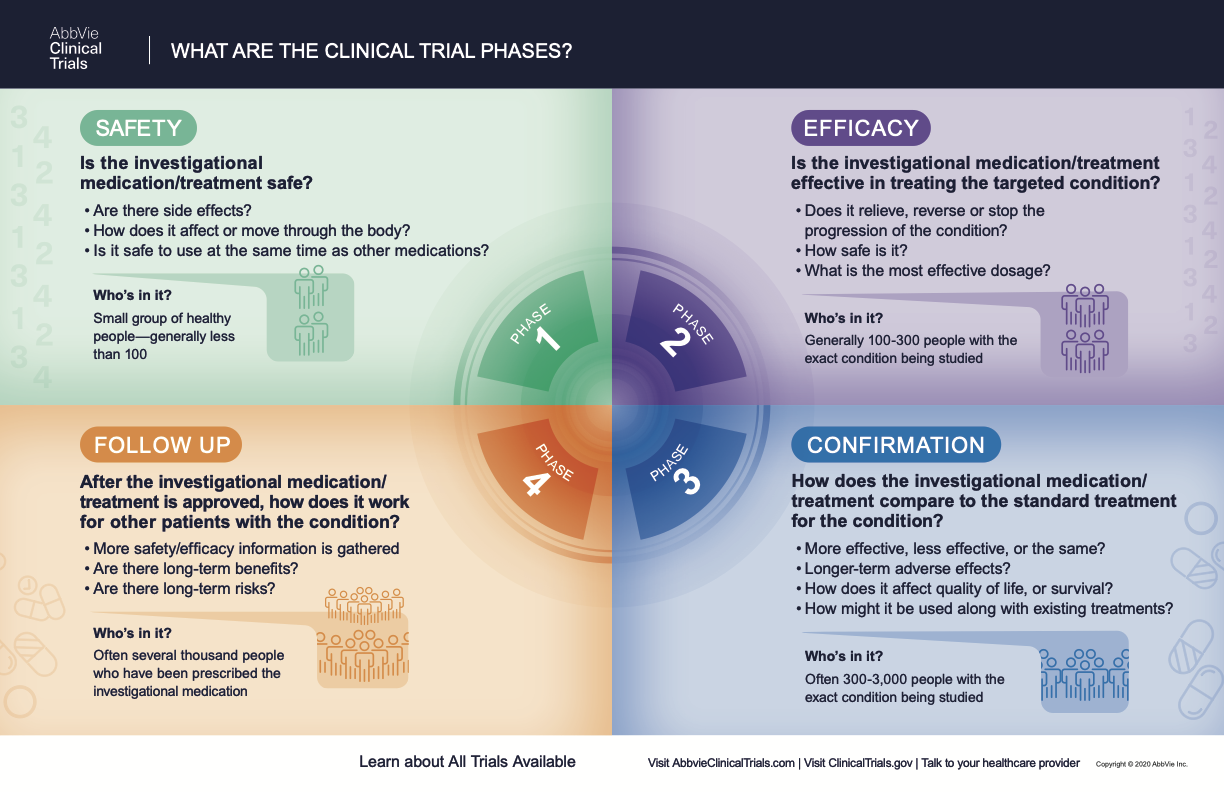
What Are The Phases Of Clinical Trials Clinical Trial Phases
What Is Medical Device Clinical Trials A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a. This review describes the fda. A clinical trial is a research study of a health product to investigate any of the following in humans: Discover or verify its clinical,. An applicable device clinical trial is a prospective clinical study of health outcomes comparing an intervention with a device against a control in. In the context of medical devices, a clinical trial or a clinical investigation can be defined as any systematic investigation or study on one or more human subjects undertaken to assess. Find out more about what is required for clinical trials of medical devices. “an instrument…intended for use in the “diagnosis…cure,. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a. As defined in section 201 of the federal food, drug, and cosmetic act, a medical device is:
From www.massdevice.com
Top 3 Reasons Why Your Medical Device Needs a Clinical Trial MassDevice What Is Medical Device Clinical Trials A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a. A clinical trial is a research study of a health product to investigate any of the following in humans: Find out more about what is required for clinical trials of medical devices. In the context of medical devices, a clinical. What Is Medical Device Clinical Trials.
From premier-research.com
7 Sponsor Responsibilities in Medical Device Clinical Trials > Premier What Is Medical Device Clinical Trials An applicable device clinical trial is a prospective clinical study of health outcomes comparing an intervention with a device against a control in. Find out more about what is required for clinical trials of medical devices. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a. A clinical trial is. What Is Medical Device Clinical Trials.
From www.youtube.com
Medical Device Clinical Trials YouTube What Is Medical Device Clinical Trials This review describes the fda. “an instrument…intended for use in the “diagnosis…cure,. Discover or verify its clinical,. As defined in section 201 of the federal food, drug, and cosmetic act, a medical device is: A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a. A clinical trial is a research. What Is Medical Device Clinical Trials.
From alldesigns.github.io
25 Good Adaptive designs for medical device clinical studies For Trend What Is Medical Device Clinical Trials A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a. This review describes the fda. “an instrument…intended for use in the “diagnosis…cure,. An applicable device clinical trial is a prospective clinical study of health outcomes comparing an intervention with a device against a control in. In the context of medical. What Is Medical Device Clinical Trials.
From prorelixresearch.com
Software as medical device clinical trials as per US FDA What Is Medical Device Clinical Trials In the context of medical devices, a clinical trial or a clinical investigation can be defined as any systematic investigation or study on one or more human subjects undertaken to assess. Find out more about what is required for clinical trials of medical devices. As defined in section 201 of the federal food, drug, and cosmetic act, a medical device. What Is Medical Device Clinical Trials.
From www.burkeins.ie
Medical Device and Clinical Trials Insurance What Is Medical Device Clinical Trials As defined in section 201 of the federal food, drug, and cosmetic act, a medical device is: “an instrument…intended for use in the “diagnosis…cure,. A clinical trial is a research study of a health product to investigate any of the following in humans: Find out more about what is required for clinical trials of medical devices. Discover or verify its. What Is Medical Device Clinical Trials.
From genesisresearchservices.com
Clinical Trials Medical Device Trials Genesis Research Services What Is Medical Device Clinical Trials Discover or verify its clinical,. An applicable device clinical trial is a prospective clinical study of health outcomes comparing an intervention with a device against a control in. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a. Find out more about what is required for clinical trials of medical. What Is Medical Device Clinical Trials.
From www.mds-foundation.org
Clinical Trials MDS Foundation What Is Medical Device Clinical Trials An applicable device clinical trial is a prospective clinical study of health outcomes comparing an intervention with a device against a control in. Discover or verify its clinical,. As defined in section 201 of the federal food, drug, and cosmetic act, a medical device is: A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses. What Is Medical Device Clinical Trials.
From www.greenlight.guru
Medical Device Clinical Trials An Overview [+Types Explained] What Is Medical Device Clinical Trials “an instrument…intended for use in the “diagnosis…cure,. As defined in section 201 of the federal food, drug, and cosmetic act, a medical device is: Find out more about what is required for clinical trials of medical devices. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a. An applicable device. What Is Medical Device Clinical Trials.
From www.mtaa.org.au
Clinical Trials for Medical Devices The Basics MTAA What Is Medical Device Clinical Trials An applicable device clinical trial is a prospective clinical study of health outcomes comparing an intervention with a device against a control in. “an instrument…intended for use in the “diagnosis…cure,. A clinical trial is a research study of a health product to investigate any of the following in humans: As defined in section 201 of the federal food, drug, and. What Is Medical Device Clinical Trials.
From www.greenlight.guru
Medical Device Adverse Event Reporting Regulations EU vs. US What Is Medical Device Clinical Trials Discover or verify its clinical,. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a. A clinical trial is a research study of a health product to investigate any of the following in humans: Find out more about what is required for clinical trials of medical devices. “an instrument…intended for. What Is Medical Device Clinical Trials.
From www.meddeviceonline.com
Managing Risk For Medical Device Clinical Trials What Is Medical Device Clinical Trials A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a. Find out more about what is required for clinical trials of medical devices. This review describes the fda. Discover or verify its clinical,. A clinical trial is a research study of a health product to investigate any of the following. What Is Medical Device Clinical Trials.
From vial.com
Exploring Medical Device Clinical Trials and their Development Pipeline What Is Medical Device Clinical Trials As defined in section 201 of the federal food, drug, and cosmetic act, a medical device is: “an instrument…intended for use in the “diagnosis…cure,. An applicable device clinical trial is a prospective clinical study of health outcomes comparing an intervention with a device against a control in. A clinical trial is a research study of a health product to investigate. What Is Medical Device Clinical Trials.
From pharpoint.com
Medical Device Clinical Trials Classification & Challenges PharPoint What Is Medical Device Clinical Trials In the context of medical devices, a clinical trial or a clinical investigation can be defined as any systematic investigation or study on one or more human subjects undertaken to assess. An applicable device clinical trial is a prospective clinical study of health outcomes comparing an intervention with a device against a control in. Find out more about what is. What Is Medical Device Clinical Trials.
From www.universityofcalifornia.edu
A new phase for clinical trials University of California What Is Medical Device Clinical Trials A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a. This review describes the fda. In the context of medical devices, a clinical trial or a clinical investigation can be defined as any systematic investigation or study on one or more human subjects undertaken to assess. “an instrument…intended for use. What Is Medical Device Clinical Trials.
From www.news-medical.net
Critical gaps found in pediatric medical device trials Lack of control What Is Medical Device Clinical Trials This review describes the fda. A clinical trial is a research study of a health product to investigate any of the following in humans: In the context of medical devices, a clinical trial or a clinical investigation can be defined as any systematic investigation or study on one or more human subjects undertaken to assess. A clinical trial is a. What Is Medical Device Clinical Trials.
From www.youtube.com
Clinical Trials in Medical Devices YouTube What Is Medical Device Clinical Trials “an instrument…intended for use in the “diagnosis…cure,. This review describes the fda. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a. In the context of medical devices, a clinical trial or a clinical investigation can be defined as any systematic investigation or study on one or more human subjects. What Is Medical Device Clinical Trials.
From www.nsmedicaldevices.com
The increasing importance of decentralised medical device trials What Is Medical Device Clinical Trials “an instrument…intended for use in the “diagnosis…cure,. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a. In the context of medical devices, a clinical trial or a clinical investigation can be defined as any systematic investigation or study on one or more human subjects undertaken to assess. Discover or. What Is Medical Device Clinical Trials.
From www.abbvieclinicaltrials.com
What Are The Phases Of Clinical Trials Clinical Trial Phases What Is Medical Device Clinical Trials In the context of medical devices, a clinical trial or a clinical investigation can be defined as any systematic investigation or study on one or more human subjects undertaken to assess. As defined in section 201 of the federal food, drug, and cosmetic act, a medical device is: A clinical trial is a systematic assessment of the device’s safety and/or. What Is Medical Device Clinical Trials.
From premier-research.com
Medical Device Trials What You Need to Know About U.S. Regulations What Is Medical Device Clinical Trials Discover or verify its clinical,. Find out more about what is required for clinical trials of medical devices. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a. In the context of medical devices, a clinical trial or a clinical investigation can be defined as any systematic investigation or study. What Is Medical Device Clinical Trials.
From www.insightsonindia.com
Insights Daily Current Affairs, 04 December 2017 INSIGHTS What Is Medical Device Clinical Trials As defined in section 201 of the federal food, drug, and cosmetic act, a medical device is: In the context of medical devices, a clinical trial or a clinical investigation can be defined as any systematic investigation or study on one or more human subjects undertaken to assess. This review describes the fda. A clinical trial is a systematic assessment. What Is Medical Device Clinical Trials.
From www.antidote.me
How do medical device clinical trials work? What Is Medical Device Clinical Trials An applicable device clinical trial is a prospective clinical study of health outcomes comparing an intervention with a device against a control in. Find out more about what is required for clinical trials of medical devices. As defined in section 201 of the federal food, drug, and cosmetic act, a medical device is: In the context of medical devices, a. What Is Medical Device Clinical Trials.
From www.youtube.com
What everybody should know about Clinical Trials! Part 3 What is a What Is Medical Device Clinical Trials Find out more about what is required for clinical trials of medical devices. Discover or verify its clinical,. As defined in section 201 of the federal food, drug, and cosmetic act, a medical device is: “an instrument…intended for use in the “diagnosis…cure,. This review describes the fda. A clinical trial is a research study of a health product to investigate. What Is Medical Device Clinical Trials.
From www.youtube.com
Clinical investigation of medical devices, regulation of What Is Medical Device Clinical Trials “an instrument…intended for use in the “diagnosis…cure,. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a. Discover or verify its clinical,. As defined in section 201 of the federal food, drug, and cosmetic act, a medical device is: Find out more about what is required for clinical trials of. What Is Medical Device Clinical Trials.
From www.greenlight.guru
Medical Device Clinical Trials An Overview [+Types Explained] What Is Medical Device Clinical Trials Find out more about what is required for clinical trials of medical devices. In the context of medical devices, a clinical trial or a clinical investigation can be defined as any systematic investigation or study on one or more human subjects undertaken to assess. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human. What Is Medical Device Clinical Trials.
From www.youtube.com
Clinical Trials for Active Medical Devices YouTube What Is Medical Device Clinical Trials A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a. “an instrument…intended for use in the “diagnosis…cure,. A clinical trial is a research study of a health product to investigate any of the following in humans: In the context of medical devices, a clinical trial or a clinical investigation can. What Is Medical Device Clinical Trials.
From diagramresearch.com
The 3 Stages For Medical Device Clinical Investigation Explained What Is Medical Device Clinical Trials Discover or verify its clinical,. Find out more about what is required for clinical trials of medical devices. A clinical trial is a research study of a health product to investigate any of the following in humans: A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a. In the context. What Is Medical Device Clinical Trials.
From www.mastercontrol.com
Medical Device Clinical Trials and Regulatory Changes MasterControl What Is Medical Device Clinical Trials An applicable device clinical trial is a prospective clinical study of health outcomes comparing an intervention with a device against a control in. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a. A clinical trial is a research study of a health product to investigate any of the following. What Is Medical Device Clinical Trials.
From www.nurseregistry.com
Clinical Trials at the Stanford Cancer Institute NurseRegistry What Is Medical Device Clinical Trials Discover or verify its clinical,. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a. In the context of medical devices, a clinical trial or a clinical investigation can be defined as any systematic investigation or study on one or more human subjects undertaken to assess. An applicable device clinical. What Is Medical Device Clinical Trials.
From premier-research.com
Considerations for the Design and Execution of Medical Device Trials What Is Medical Device Clinical Trials As defined in section 201 of the federal food, drug, and cosmetic act, a medical device is: An applicable device clinical trial is a prospective clinical study of health outcomes comparing an intervention with a device against a control in. In the context of medical devices, a clinical trial or a clinical investigation can be defined as any systematic investigation. What Is Medical Device Clinical Trials.
From www.jli.edu.in
5 Phases of Medical Device Development Process JLI Blog What Is Medical Device Clinical Trials An applicable device clinical trial is a prospective clinical study of health outcomes comparing an intervention with a device against a control in. A clinical trial is a research study of a health product to investigate any of the following in humans: Find out more about what is required for clinical trials of medical devices. This review describes the fda.. What Is Medical Device Clinical Trials.
From www.biopharmaservices.com
How to Conduct Human Clinical Trials BioPharma Services What Is Medical Device Clinical Trials As defined in section 201 of the federal food, drug, and cosmetic act, a medical device is: Discover or verify its clinical,. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a. In the context of medical devices, a clinical trial or a clinical investigation can be defined as any. What Is Medical Device Clinical Trials.
From www.greenlight.guru
Medical Device Clinical Trials Regulatory Pathways & Study Types Explained What Is Medical Device Clinical Trials This review describes the fda. As defined in section 201 of the federal food, drug, and cosmetic act, a medical device is: Find out more about what is required for clinical trials of medical devices. A clinical trial is a research study of a health product to investigate any of the following in humans: “an instrument…intended for use in the. What Is Medical Device Clinical Trials.
From www.techsollifesciences.com
Medical Devices Clinical Studies / Trials Best Practices Techsol What Is Medical Device Clinical Trials An applicable device clinical trial is a prospective clinical study of health outcomes comparing an intervention with a device against a control in. Find out more about what is required for clinical trials of medical devices. A clinical trial is a research study of a health product to investigate any of the following in humans: A clinical trial is a. What Is Medical Device Clinical Trials.
From prorelixresearch.com
Medical device clinical trials and regulations in India ProRelix Research What Is Medical Device Clinical Trials “an instrument…intended for use in the “diagnosis…cure,. A clinical trial is a research study of a health product to investigate any of the following in humans: In the context of medical devices, a clinical trial or a clinical investigation can be defined as any systematic investigation or study on one or more human subjects undertaken to assess. Find out more. What Is Medical Device Clinical Trials.