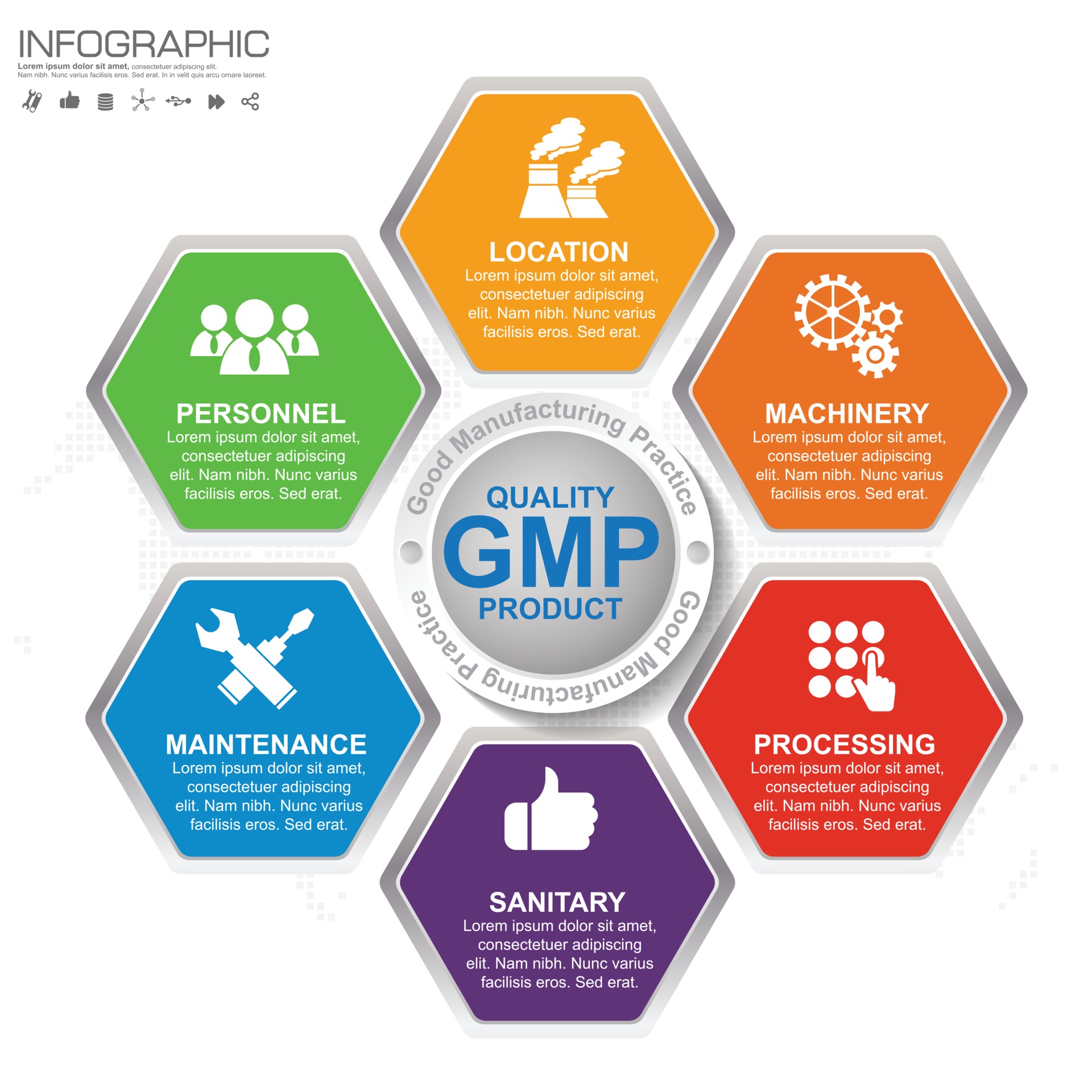
Gmp Experience Definition . good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. gmp (good manufacturing practice) is the part of quality assurance that ensures consistent quality standards in the production and testing of medicinal. good manufacturing practice (gmp) describes a set of principles and procedures that when followed helps. cgmp refers to the current good manufacturing practice regulations enforced by the fda. gmp refers to the good manufacturing practice regulations promulgated by the us food and drug administration under the. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in.
from www.vecteezy.com
good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. good manufacturing practice (gmp) describes a set of principles and procedures that when followed helps. cgmp refers to the current good manufacturing practice regulations enforced by the fda. gmp refers to the good manufacturing practice regulations promulgated by the us food and drug administration under the. gmp (good manufacturing practice) is the part of quality assurance that ensures consistent quality standards in the production and testing of medicinal.
GMP Good Manufacturing Practice 6 heading of infographic template with
Gmp Experience Definition good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. good manufacturing practice (gmp) describes a set of principles and procedures that when followed helps. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. gmp refers to the good manufacturing practice regulations promulgated by the us food and drug administration under the. cgmp refers to the current good manufacturing practice regulations enforced by the fda. gmp (good manufacturing practice) is the part of quality assurance that ensures consistent quality standards in the production and testing of medicinal.
From marketbusinessnews.com
Good manufacturing practice definition and meaning Gmp Experience Definition good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. cgmp refers to the current good manufacturing practice regulations enforced by the fda. good manufacturing practice (gmp) describes a set of. Gmp Experience Definition.
From blog.nbs-us.com
What is GMP, and Why is it Important for Life Sciences Firms? Gmp Experience Definition good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. gmp (good manufacturing practice) is the part of quality assurance that ensures consistent quality standards in the production and testing of medicinal.. Gmp Experience Definition.
From www.vetter-pharma.com
cGMP vs. GMP What they are and how they differ Gmp Experience Definition gmp (good manufacturing practice) is the part of quality assurance that ensures consistent quality standards in the production and testing of medicinal. good manufacturing practice (gmp) describes a set of principles and procedures that when followed helps. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards.. Gmp Experience Definition.
From www.cbmmaryland.com
The 10 Principles of GMP Commercial Building Maintenance Gmp Experience Definition good manufacturing practice (gmp) describes a set of principles and procedures that when followed helps. gmp refers to the good manufacturing practice regulations promulgated by the us food and drug administration under the. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. good manufacturing practice (gmp) is a system for. Gmp Experience Definition.
From www.vecteezy.com
GMP Good Manufacturing Practice 6 heading of infographic template with Gmp Experience Definition good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. gmp refers to the good manufacturing practice regulations promulgated by the us food and drug administration under the. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. gmp (good manufacturing. Gmp Experience Definition.
From www.slideserve.com
PPT REVISION OF EUDRALEX VOL. 4 GMP Luisa Stoppa, Ph.D. Inspection Gmp Experience Definition cgmp refers to the current good manufacturing practice regulations enforced by the fda. gmp refers to the good manufacturing practice regulations promulgated by the us food and drug administration under the. gmp (good manufacturing practice) is the part of quality assurance that ensures consistent quality standards in the production and testing of medicinal. good manufacturing practice. Gmp Experience Definition.
From www.ppd.com
GMP Lab Experience and Expertise PPD Gmp Experience Definition gmp refers to the good manufacturing practice regulations promulgated by the us food and drug administration under the. good manufacturing practice (gmp) describes a set of principles and procedures that when followed helps. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. gmp (good manufacturing practice) is the part of. Gmp Experience Definition.
From www.slideserve.com
PPT REVISION OF EUDRALEX VOL. 4 GMP Luisa Stoppa, Ph.D. Inspection Gmp Experience Definition cgmp refers to the current good manufacturing practice regulations enforced by the fda. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. good manufacturing practice (gmp) describes a set of principles and procedures that when followed helps. gmp refers to the good manufacturing practice regulations. Gmp Experience Definition.
From www.scribd.com
GMP AND cGMP CONSIDERATIONS Quality Assurance Pharmacy Gmp Experience Definition gmp refers to the good manufacturing practice regulations promulgated by the us food and drug administration under the. cgmp refers to the current good manufacturing practice regulations enforced by the fda. good manufacturing practice (gmp) describes a set of principles and procedures that when followed helps. good manufacturing practice (gmp) describes the minimum standard that a. Gmp Experience Definition.
From www.pharmaguideline.co.uk
Definition of GMP according to different Pharmaceutical guideline Gmp Experience Definition cgmp refers to the current good manufacturing practice regulations enforced by the fda. gmp refers to the good manufacturing practice regulations promulgated by the us food and drug administration under the. gmp (good manufacturing practice) is the part of quality assurance that ensures consistent quality standards in the production and testing of medicinal. good manufacturing practice. Gmp Experience Definition.
From www.pharmaaccess.net
The GMP Framework Pharmaaccess Gmp Experience Definition good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. good manufacturing practice (gmp) describes a set of principles and procedures that when followed helps. cgmp refers to the current good. Gmp Experience Definition.
From www.gmp-online.com
GMP Experience Good Manufacturing Practices Gmp Experience Definition gmp (good manufacturing practice) is the part of quality assurance that ensures consistent quality standards in the production and testing of medicinal. gmp refers to the good manufacturing practice regulations promulgated by the us food and drug administration under the. cgmp refers to the current good manufacturing practice regulations enforced by the fda. good manufacturing practice. Gmp Experience Definition.
From exopftayl.blob.core.windows.net
Gmp Process Validation Protocol at Jeanne McElwee blog Gmp Experience Definition cgmp refers to the current good manufacturing practice regulations enforced by the fda. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. gmp refers to the good manufacturing practice regulations promulgated by the us food and drug administration under the. gmp (good manufacturing practice) is the part of quality assurance. Gmp Experience Definition.
From www.vrogue.co
Was Ist Gmp Good Manufacturing Practice Definition Gm vrogue.co Gmp Experience Definition cgmp refers to the current good manufacturing practice regulations enforced by the fda. good manufacturing practice (gmp) describes a set of principles and procedures that when followed helps. gmp (good manufacturing practice) is the part of quality assurance that ensures consistent quality standards in the production and testing of medicinal. good manufacturing practice (gmp) is a. Gmp Experience Definition.
From www.slideserve.com
PPT Good Manufacturing Practices Purpose and Principles of GMP Gmp Experience Definition good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. cgmp refers to the current good manufacturing practice regulations enforced by the fda. good manufacturing practice (gmp) describes a set of. Gmp Experience Definition.
From www.pharmaspecialists.com
Basic Principles of GMP in Pharmaceutical Industry Gmp Experience Definition good manufacturing practice (gmp) describes a set of principles and procedures that when followed helps. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. cgmp refers to the current good manufacturing practice regulations enforced by the fda. good manufacturing practice (gmp) is a system for ensuring that products are consistently. Gmp Experience Definition.
From flairpharma.com
GMP Audit Checklist » Flair Pharma The Knowledge Kit. Gmp Experience Definition cgmp refers to the current good manufacturing practice regulations enforced by the fda. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. gmp (good manufacturing practice) is the part of quality assurance that ensures consistent quality standards in the production and testing of medicinal. good. Gmp Experience Definition.
From isolocity.com
The Importance of GMPValidated QMS for Manufacturing Industry Gmp Experience Definition cgmp refers to the current good manufacturing practice regulations enforced by the fda. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. gmp refers to the good manufacturing practice regulations. Gmp Experience Definition.
From www.youtube.com
Important of GMP in Pharmaceutical Industry Part 01 YouTube Gmp Experience Definition good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. good manufacturing practice (gmp) describes a set of principles and procedures that when followed helps. gmp (good manufacturing practice) is the. Gmp Experience Definition.
From www.compliancequest.com
What is GMP Quality? GMP Standards and Regulations Gmp Experience Definition gmp (good manufacturing practice) is the part of quality assurance that ensures consistent quality standards in the production and testing of medicinal. gmp refers to the good manufacturing practice regulations promulgated by the us food and drug administration under the. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according. Gmp Experience Definition.
From www.linkedin.com
GMP Compliance 101 Understanding the Purpose and Significance of GMP Gmp Experience Definition good manufacturing practice (gmp) describes a set of principles and procedures that when followed helps. gmp refers to the good manufacturing practice regulations promulgated by the us food and drug administration under the. gmp (good manufacturing practice) is the part of quality assurance that ensures consistent quality standards in the production and testing of medicinal. good. Gmp Experience Definition.
From www.gmp-publishing.com
What is GMP? Good Manufacturing Practice Definition GMPVerlag Gmp Experience Definition gmp refers to the good manufacturing practice regulations promulgated by the us food and drug administration under the. good manufacturing practice (gmp) describes a set of principles and procedures that when followed helps. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. gmp (good manufacturing. Gmp Experience Definition.
From pediaa.com
Difference Between GMP and cGMP Gmp Experience Definition good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. gmp refers to the good manufacturing practice regulations promulgated by the us food and drug administration under the. cgmp refers to the current good manufacturing practice regulations enforced by the fda. good manufacturing practice (gmp) is a system for ensuring that. Gmp Experience Definition.
From www.slideserve.com
PPT REVISION OF EUDRALEX VOL. 4 GMP Luisa Stoppa, Ph.D. Inspection Gmp Experience Definition good manufacturing practice (gmp) describes a set of principles and procedures that when followed helps. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. gmp refers to the good manufacturing. Gmp Experience Definition.
From www.iowalum.com
What is GMP? Gmp Experience Definition good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. cgmp refers to the current good manufacturing practice regulations enforced by the fda. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. gmp refers to the good manufacturing practice regulations. Gmp Experience Definition.
From www.slideserve.com
PPT REVISION OF EUDRALEX VOL. 4 GMP Luisa Stoppa, Ph.D. Inspection Gmp Experience Definition cgmp refers to the current good manufacturing practice regulations enforced by the fda. gmp refers to the good manufacturing practice regulations promulgated by the us food and drug administration under the. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. good manufacturing practice (gmp) describes a set of principles and. Gmp Experience Definition.
From www.arenasolutions.com
Good Manufacturing Practice (GMP ) Definition Arena Gmp Experience Definition good manufacturing practice (gmp) describes a set of principles and procedures that when followed helps. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. cgmp refers to the current good. Gmp Experience Definition.
From intracoenc.com
Good Manufacturing Practices (GMP) 101 Definition and Guidelines Gmp Experience Definition gmp refers to the good manufacturing practice regulations promulgated by the us food and drug administration under the. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. good manufacturing practice (gmp) describes a set of principles and procedures that when followed helps. gmp (good manufacturing practice) is the part of. Gmp Experience Definition.
From www.vectorstock.com
Gmpgood manufacturing practice 6 heading Vector Image Gmp Experience Definition gmp refers to the good manufacturing practice regulations promulgated by the us food and drug administration under the. good manufacturing practice (gmp) describes a set of principles and procedures that when followed helps. gmp (good manufacturing practice) is the part of quality assurance that ensures consistent quality standards in the production and testing of medicinal. cgmp. Gmp Experience Definition.
From www.vecteezy.com
GMP Good Manufacturing Practice 6 heading of infographic template with Gmp Experience Definition cgmp refers to the current good manufacturing practice regulations enforced by the fda. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. gmp (good manufacturing practice) is the part of quality assurance that ensures consistent quality standards in the production and testing of medicinal. gmp. Gmp Experience Definition.
From www.scilife.io
What are the 5 Main Components of GMP? Full definition Scilife Gmp Experience Definition good manufacturing practice (gmp) describes a set of principles and procedures that when followed helps. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. cgmp refers to the current good manufacturing practice regulations enforced by the fda. gmp refers to the good manufacturing practice regulations. Gmp Experience Definition.
From www.youtube.com
GMP 101 Intro to Good Manufacturing Practice [WEBINAR] YouTube Gmp Experience Definition good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. gmp (good manufacturing practice) is the part of quality assurance that ensures consistent quality standards in the production and testing of medicinal. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in.. Gmp Experience Definition.
From www.etsy.com
General Good Manufacturing Practices GMP TEMPLATES for Manufacturing Etsy Gmp Experience Definition cgmp refers to the current good manufacturing practice regulations enforced by the fda. good manufacturing practice (gmp) describes a set of principles and procedures that when followed helps. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. good manufacturing practice (gmp) describes the minimum standard. Gmp Experience Definition.
From www.slideshare.net
GMP AND cGMP CONSIDERATIONS Gmp Experience Definition good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. good manufacturing practice (gmp) describes a set of principles and procedures that when followed helps. good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in. gmp refers to the good manufacturing. Gmp Experience Definition.
From www.compliance-insight.com
The Ten Core Principles of GMP Compliance Compliance Insight Gmp Experience Definition gmp (good manufacturing practice) is the part of quality assurance that ensures consistent quality standards in the production and testing of medicinal. gmp refers to the good manufacturing practice regulations promulgated by the us food and drug administration under the. good manufacturing practice (gmp) describes a set of principles and procedures that when followed helps. good. Gmp Experience Definition.