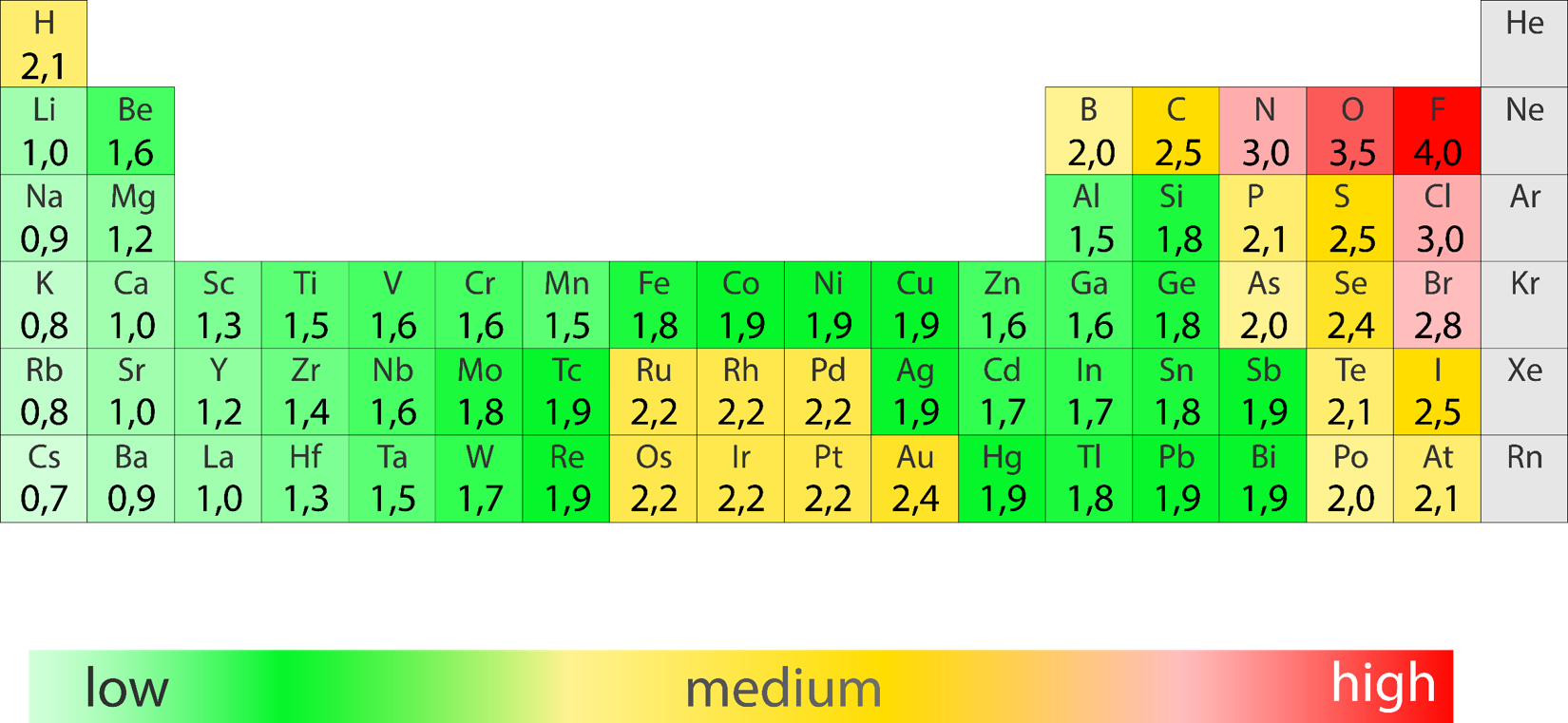
Aluminum Fluoride Electronegativity Difference . The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. Fluorine is highly electronegative, causing the aluminum atom to carry a slight positive charge. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. The pauling scale is the most commonly used. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself.
from mungfali.com
Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. The pauling scale is the most commonly used. Fluorine is highly electronegative, causing the aluminum atom to carry a slight positive charge. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values.
Electronegativity And Ionization Energy
Aluminum Fluoride Electronegativity Difference The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. The pauling scale is the most commonly used. Fluorine is highly electronegative, causing the aluminum atom to carry a slight positive charge. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values.
From southernplantationstylefurniture.blogspot.com
The Periodic Table Trend Of Electronegativity Follows What Pattern Aluminum Fluoride Electronegativity Difference Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. The pauling scale is the most commonly used. Fluorine is highly electronegative, causing the aluminum atom to carry a slight positive charge. When two. Aluminum Fluoride Electronegativity Difference.
From www.ck12.org
Periodic Trends in Electronegativity CK12 Foundation Aluminum Fluoride Electronegativity Difference The pauling scale is the most commonly used. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity. Aluminum Fluoride Electronegativity Difference.
From www.slideshare.net
6 Electronegativity And Electron Affinity Aluminum Fluoride Electronegativity Difference Fluorine is highly electronegative, causing the aluminum atom to carry a slight positive charge. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. The electronegativity calculator allows you to calculate the type. Aluminum Fluoride Electronegativity Difference.
From mungfali.com
Electronegativity Trend Explained Aluminum Fluoride Electronegativity Difference The pauling scale is the most commonly used. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of. Aluminum Fluoride Electronegativity Difference.
From mungfali.com
Electronegativity And Ionization Energy Aluminum Fluoride Electronegativity Difference Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The pauling scale is the most commonly used. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Fluorine is highly electronegative, causing the aluminum atom to carry a slight positive charge. When two. Aluminum Fluoride Electronegativity Difference.
From anthonystrendsassignment.weebly.com
Electronegativity Periodic Trends Aluminum Fluoride Electronegativity Difference Fluorine is highly electronegative, causing the aluminum atom to carry a slight positive charge. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The pauling scale is the most commonly used. The bond polarity between. Aluminum Fluoride Electronegativity Difference.
From www.adda247.com
Aluminium Fluoride Formula Structure, Chemical Name, Uses Aluminum Fluoride Electronegativity Difference The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Electronegativity is a chemical. Aluminum Fluoride Electronegativity Difference.
From mavink.com
Periodic Table With Electronegativity Values Aluminum Fluoride Electronegativity Difference The pauling scale is the most commonly used. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Fluorine is highly electronegative, causing the aluminum atom to carry a slight positive charge. When two atoms combine,. Aluminum Fluoride Electronegativity Difference.
From courses.lumenlearning.com
4.4 Characteristics of Covalent Bonds The Basics of General, Organic Aluminum Fluoride Electronegativity Difference Fluorine is highly electronegative, causing the aluminum atom to carry a slight positive charge. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The electronegativity calculator allows you to calculate the type. Aluminum Fluoride Electronegativity Difference.
From www.adda247.com
Aluminium Fluoride Formula Structure, Chemical Name, Uses Aluminum Fluoride Electronegativity Difference The pauling scale is the most commonly used. Fluorine is highly electronegative, causing the aluminum atom to carry a slight positive charge. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. The bond. Aluminum Fluoride Electronegativity Difference.
From www.vrogue.co
Printable Electronegativity Periodic Table Electroneg vrogue.co Aluminum Fluoride Electronegativity Difference Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Fluorine is highly electronegative, causing the aluminum. Aluminum Fluoride Electronegativity Difference.
From mungfali.com
Electronegativity Difference Chart Aluminum Fluoride Electronegativity Difference The pauling scale is the most commonly used. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity. Aluminum Fluoride Electronegativity Difference.
From mavink.com
Electronegativity Difference Chart Aluminum Fluoride Electronegativity Difference When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Fluorine is highly electronegative, causing the aluminum atom to carry a slight positive charge. Electronegativity is a chemical property which describes how well. Aluminum Fluoride Electronegativity Difference.
From www.thoughtco.com
Printable Periodic Table of the Elements Electronegativity Aluminum Fluoride Electronegativity Difference Fluorine is highly electronegative, causing the aluminum atom to carry a slight positive charge. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Electronegativity is a chemical property which describes how well an. Aluminum Fluoride Electronegativity Difference.
From www.aiophotoz.com
Periodic Table Of Elements Electronegativity Chart Periodic Table Aluminum Fluoride Electronegativity Difference The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. The pauling scale is the most commonly used. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. When two atoms combine, the difference between their electronegativities is an indication of the type of. Aluminum Fluoride Electronegativity Difference.
From mungfali.com
Electronegativity Difference Chart Aluminum Fluoride Electronegativity Difference When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. The bond polarity between. Aluminum Fluoride Electronegativity Difference.
From sciencetrends.com
Electronegativity Trend Science Trends Aluminum Fluoride Electronegativity Difference The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. The pauling scale is the most commonly used. Fluorine is highly electronegative, causing the aluminum atom to carry a slight positive charge. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. The. Aluminum Fluoride Electronegativity Difference.
From mungfali.com
Electronegativity Chart And Lewis Structures Aluminum Fluoride Electronegativity Difference The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Fluorine is highly electronegative, causing the aluminum atom to carry a slight positive charge. Electronegativity is a chemical property which describes how well an atom can. Aluminum Fluoride Electronegativity Difference.
From iperiodictable.com
Periodic Table Electronegativity Periodic Table Aluminum Fluoride Electronegativity Difference Fluorine is highly electronegative, causing the aluminum atom to carry a slight positive charge. The pauling scale is the most commonly used. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The bond polarity between. Aluminum Fluoride Electronegativity Difference.
From mungfali.com
Electronegativity Difference Chart Aluminum Fluoride Electronegativity Difference Fluorine is highly electronegative, causing the aluminum atom to carry a slight positive charge. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The pauling scale is the most commonly used. The. Aluminum Fluoride Electronegativity Difference.
From reviewhomedecor.co
Chemistry Reference Table Electronegativity Review Home Decor Aluminum Fluoride Electronegativity Difference The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The pauling scale is the most commonly used. When two atoms combine, the difference between their electronegativities is an indication of the type of. Aluminum Fluoride Electronegativity Difference.
From www.animalia-life.club
Electronegativity Difference Bond Type Aluminum Fluoride Electronegativity Difference When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. The pauling scale is the most commonly used. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Fluorine is highly electronegative, causing the aluminum atom to carry a slight positive charge. Electronegativity. Aluminum Fluoride Electronegativity Difference.
From dona.tompkinscountystructuralracism.org
Electronegativity Values Chart Understanding The Chemistry Of Elements Aluminum Fluoride Electronegativity Difference Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. When two atoms combine, the difference between. Aluminum Fluoride Electronegativity Difference.
From mungfali.com
Electronegativity Difference Chart Aluminum Fluoride Electronegativity Difference The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Fluorine is highly electronegative, causing the aluminum atom to carry a slight positive charge. When two atoms combine, the difference between their electronegativities is an indication. Aluminum Fluoride Electronegativity Difference.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Aluminum Fluoride Electronegativity Difference Fluorine is highly electronegative, causing the aluminum atom to carry a slight positive charge. The pauling scale is the most commonly used. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. When two. Aluminum Fluoride Electronegativity Difference.
From www.animalia-life.club
Electronegativity Difference Bond Type Aluminum Fluoride Electronegativity Difference The pauling scale is the most commonly used. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Fluorine is highly electronegative, causing the aluminum atom to carry a slight positive charge. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The bond polarity between. Aluminum Fluoride Electronegativity Difference.
From pubchem.ncbi.nlm.nih.gov
Electronegativity Periodic Table of Elements PubChem Aluminum Fluoride Electronegativity Difference The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The pauling scale is the most commonly. Aluminum Fluoride Electronegativity Difference.
From ar.inspiredpencil.com
Electronegativity List Aluminum Fluoride Electronegativity Difference The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Electronegativity is a chemical. Aluminum Fluoride Electronegativity Difference.
From mungfali.com
Electronegativity Difference Chart Aluminum Fluoride Electronegativity Difference The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. The pauling scale is the most commonly used. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of. Aluminum Fluoride Electronegativity Difference.
From slideplayer.com
Chemical Bonding. ppt download Aluminum Fluoride Electronegativity Difference Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The pauling scale is the most commonly used. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity. Aluminum Fluoride Electronegativity Difference.
From culturevie.info
Electronegativity Chart Aluminum Fluoride Electronegativity Difference The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. The pauling scale is the most commonly used. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Electronegativity is a chemical property which describes how well an atom can attract an electron to. Aluminum Fluoride Electronegativity Difference.
From chemdictionary.org
Electronegativity Definition And Examples Chemistry Dictionary Aluminum Fluoride Electronegativity Difference Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The pauling scale is the most commonly used. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. The electronegativity calculator allows you to calculate the type of bond formed between different elements. Aluminum Fluoride Electronegativity Difference.
From www.nagwa.com
Question Video Identifying Which Element Has a Similar Aluminum Fluoride Electronegativity Difference Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The electronegativity calculator allows you to. Aluminum Fluoride Electronegativity Difference.
From animalia-life.club
Electronegativity Difference Bond Type Aluminum Fluoride Electronegativity Difference Fluorine is highly electronegative, causing the aluminum atom to carry a slight positive charge. The pauling scale is the most commonly used. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. When two atoms combine,. Aluminum Fluoride Electronegativity Difference.
From atonce.com
50 Unveiled Trends in Electronegativity & First Ionization Ultimate Aluminum Fluoride Electronegativity Difference Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The pauling scale is the most commonly used. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that. Aluminum Fluoride Electronegativity Difference.