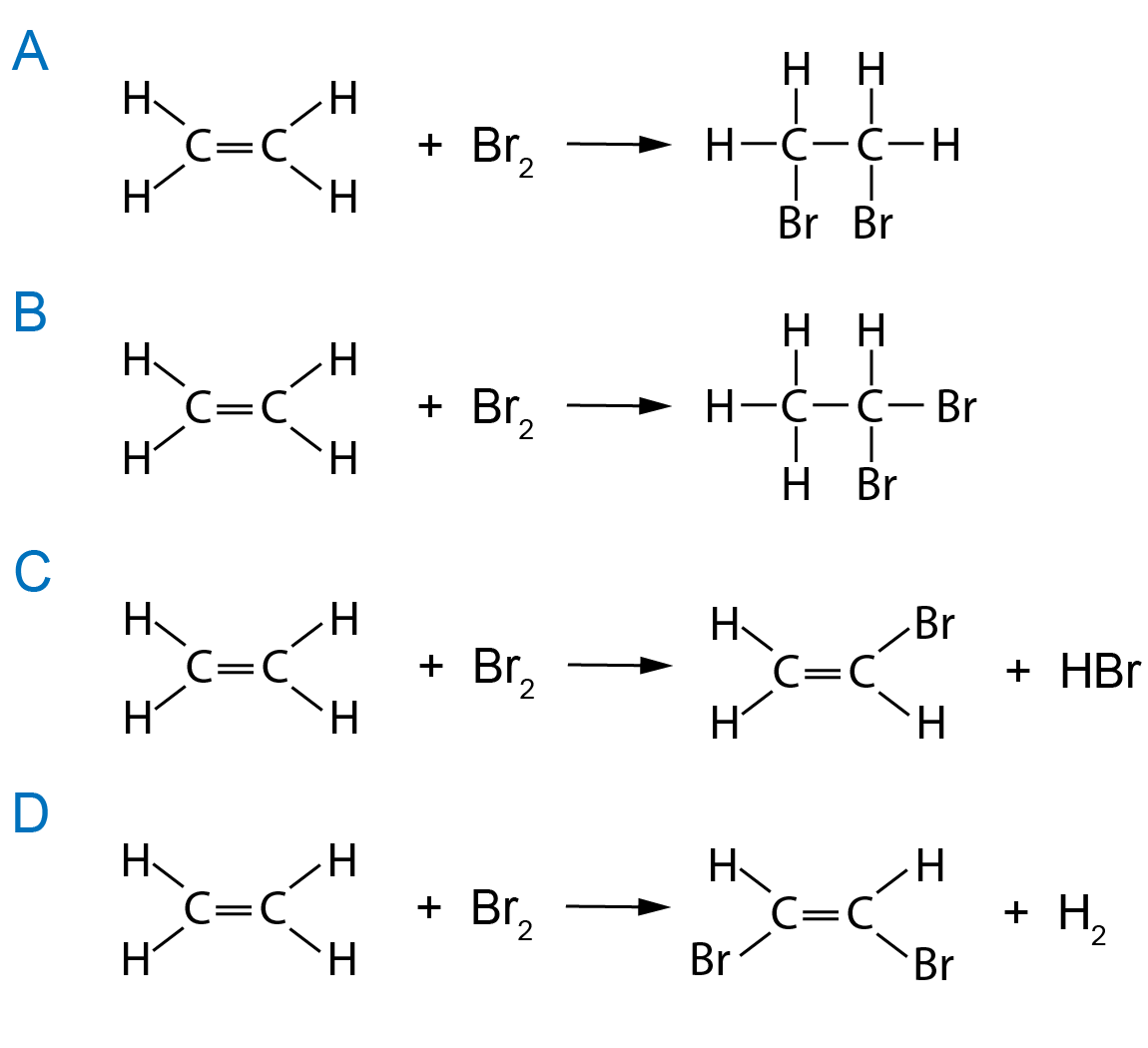Bromine Product Formula . Bromine is mainly used to produce bromides. The multiple active ingredient products control. As an important chemical raw material, it is widely used in. It has an atomic weight of 79.904 and a mass. Two products with multiple active ingredients and two products as the sole active ingredient. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The free bromine is then mixed with sulfur dioxide, and the. Bromine is an active ingredient in four products; The image intends to reflect the rich colour, liquidity and aromatic nature of the element. The product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it.
from gradegorilla.com
It has an atomic weight of 79.904 and a mass. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Two products with multiple active ingredients and two products as the sole active ingredient. As an important chemical raw material, it is widely used in. The free bromine is then mixed with sulfur dioxide, and the. Bromine is mainly used to produce bromides. Bromine is an active ingredient in four products; The multiple active ingredient products control. The product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. The image intends to reflect the rich colour, liquidity and aromatic nature of the element.
GCSE Chemistry Organic Grade Gorilla
Bromine Product Formula Bromine is mainly used to produce bromides. The multiple active ingredient products control. The image intends to reflect the rich colour, liquidity and aromatic nature of the element. Two products with multiple active ingredients and two products as the sole active ingredient. As an important chemical raw material, it is widely used in. It has an atomic weight of 79.904 and a mass. Bromine is an active ingredient in four products; The free bromine is then mixed with sulfur dioxide, and the. The product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Bromine is mainly used to produce bromides.
From www.dreamstime.com
Elemental Bromine Br2, Molecule. Skeletal Formula Stock Vector Bromine Product Formula Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The free bromine is then mixed with sulfur dioxide, and the. As an important chemical raw material, it is widely used in. It has an atomic weight of 79.904 and a mass. Bromine is an active ingredient in four products;. Bromine Product Formula.
From www.alamy.com
Bromine molecular model Stock Vector Images Alamy Bromine Product Formula Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The image intends to reflect the rich colour, liquidity and aromatic nature of the element. The product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. The multiple active. Bromine Product Formula.
From upberi.com
Bromine Formula \({\rm{B}}{{\rm{r}}_2}\) Structure, Molar Mass, IUPAC Bromine Product Formula Bromine is mainly used to produce bromides. It has an atomic weight of 79.904 and a mass. Bromine is an active ingredient in four products; The image intends to reflect the rich colour, liquidity and aromatic nature of the element. As an important chemical raw material, it is widely used in. The product of the reaction is a dilute solution. Bromine Product Formula.
From www.dreamstime.com
Bromine, Br, Periodic Table Element Stock Illustration Illustration Bromine Product Formula Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. The multiple active ingredient products control. It has an atomic weight of 79.904 and a mass. The image. Bromine Product Formula.
From www.dreamstime.com
Elemental Bromine Br2, Molecule. Skeletal Formula Stock Vector Bromine Product Formula As an important chemical raw material, it is widely used in. Bromine is mainly used to produce bromides. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Two products with multiple active ingredients and two products as the sole active ingredient. The free bromine is then mixed with sulfur. Bromine Product Formula.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Bromine Product Formula Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. As an important chemical raw material, it is widely used in. The free bromine is then mixed with. Bromine Product Formula.
From www.alamy.com
Bromine molecule, illustration Stock Photo Alamy Bromine Product Formula The image intends to reflect the rich colour, liquidity and aromatic nature of the element. Bromine is an active ingredient in four products; As an important chemical raw material, it is widely used in. Two products with multiple active ingredients and two products as the sole active ingredient. Bromine is the 35th element in the periodic table and has a. Bromine Product Formula.
From www.echemi.com
An alkene with molecular formula C4H8 reacts with bromine to form a Bromine Product Formula As an important chemical raw material, it is widely used in. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. The image intends to reflect the rich. Bromine Product Formula.
From ar.inspiredpencil.com
Bromine Molecule Bromine Product Formula Two products with multiple active ingredients and two products as the sole active ingredient. The product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. Bromine is an active ingredient in four products; The multiple active ingredient products control. It has an atomic weight of 79.904 and a mass.. Bromine Product Formula.
From gradegorilla.com
GCSE Chemistry Organic Grade Gorilla Bromine Product Formula Bromine is mainly used to produce bromides. Two products with multiple active ingredients and two products as the sole active ingredient. The image intends to reflect the rich colour, liquidity and aromatic nature of the element. The free bromine is then mixed with sulfur dioxide, and the. As an important chemical raw material, it is widely used in. Bromine is. Bromine Product Formula.
From www.alamy.com
Bromine. Bromum. Halogens. Chemical Element of Mendeleev's Periodic Bromine Product Formula The free bromine is then mixed with sulfur dioxide, and the. It has an atomic weight of 79.904 and a mass. As an important chemical raw material, it is widely used in. The multiple active ingredient products control. The product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it.. Bromine Product Formula.
From www.slideserve.com
PPT Edexcel organic reaction mechanisms PowerPoint Presentation, free Bromine Product Formula The multiple active ingredient products control. It has an atomic weight of 79.904 and a mass. The free bromine is then mixed with sulfur dioxide, and the. The image intends to reflect the rich colour, liquidity and aromatic nature of the element. Bromine is an active ingredient in four products; Bromine is the 35th element in the periodic table and. Bromine Product Formula.
From www.britannica.com
Bromine Properties, Uses, & Facts Britannica Bromine Product Formula The product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. As an important chemical raw material, it is widely used in. The free bromine is then mixed with sulfur dioxide, and the. Bromine is mainly used to produce bromides. Bromine is an active ingredient in four products; It. Bromine Product Formula.
From material-properties.org
Bromine Protons Neutrons Electrons Electron Configuration Bromine Product Formula The image intends to reflect the rich colour, liquidity and aromatic nature of the element. As an important chemical raw material, it is widely used in. The product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. Bromine is an active ingredient in four products; The free bromine is. Bromine Product Formula.
From www.alamy.com
Elemental bromine molecule Black and White Stock Photos & Images Alamy Bromine Product Formula The multiple active ingredient products control. The image intends to reflect the rich colour, liquidity and aromatic nature of the element. Two products with multiple active ingredients and two products as the sole active ingredient. Bromine is an active ingredient in four products; The free bromine is then mixed with sulfur dioxide, and the. Bromine is the 35th element in. Bromine Product Formula.
From www.fishersci.com
Bromine (BromideBromate), 0.1N Volumetric Solution, BAKER ANALYZED Bromine Product Formula Bromine is mainly used to produce bromides. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The image intends to reflect the rich colour, liquidity and aromatic nature of the element. It has an atomic weight of 79.904 and a mass. The free bromine is then mixed with sulfur. Bromine Product Formula.
From www.vecteezy.com
Bromine symbol. Chemical element of the periodic table. Vector Bromine Product Formula The free bromine is then mixed with sulfur dioxide, and the. Bromine is an active ingredient in four products; The image intends to reflect the rich colour, liquidity and aromatic nature of the element. It has an atomic weight of 79.904 and a mass. The multiple active ingredient products control. Bromine is the 35th element in the periodic table and. Bromine Product Formula.
From www.masterorganicchemistry.com
Bromination of Alkenes Master Organic Chemistry Bromine Product Formula Bromine is mainly used to produce bromides. The product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. Two products with multiple active ingredients and two products as the sole active ingredient. Bromine is the 35th element in the periodic table and has a symbol of br and atomic. Bromine Product Formula.
From ar.inspiredpencil.com
Bromine Liquid Formula Bromine Product Formula It has an atomic weight of 79.904 and a mass. The image intends to reflect the rich colour, liquidity and aromatic nature of the element. The free bromine is then mixed with sulfur dioxide, and the. The multiple active ingredient products control. Bromine is mainly used to produce bromides. Bromine is an active ingredient in four products; As an important. Bromine Product Formula.
From testbook.com
Bromine Formula Learn Definition, Formula, Structure and Uses Bromine Product Formula It has an atomic weight of 79.904 and a mass. As an important chemical raw material, it is widely used in. Bromine is an active ingredient in four products; The product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. Two products with multiple active ingredients and two products. Bromine Product Formula.
From www.vectorstock.com
Br2 bromine molecule Royalty Free Vector Image Bromine Product Formula The image intends to reflect the rich colour, liquidity and aromatic nature of the element. It has an atomic weight of 79.904 and a mass. The product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. Bromine is mainly used to produce bromides. Two products with multiple active ingredients. Bromine Product Formula.
From www.youtube.com
How to Write the Formula for Bromine Liquid YouTube Bromine Product Formula The multiple active ingredient products control. Bromine is mainly used to produce bromides. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. It has an atomic weight of 79.904 and a mass. Two products with multiple active ingredients and two products as the sole active ingredient. The free bromine. Bromine Product Formula.
From www.shutterstock.com
Reacción del bromo con estructura química vector de stock (libre de Bromine Product Formula The product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. Bromine is an active ingredient in four products; As an important chemical raw material, it is widely used in. The multiple active ingredient products control. Bromine is the 35th element in the periodic table and has a symbol. Bromine Product Formula.
From www.masterorganicchemistry.com
Bromination of alkenes with Br2 to give dibromides Master Organic Bromine Product Formula Two products with multiple active ingredients and two products as the sole active ingredient. As an important chemical raw material, it is widely used in. The free bromine is then mixed with sulfur dioxide, and the. Bromine is an active ingredient in four products; Bromine is mainly used to produce bromides. The multiple active ingredient products control. The product of. Bromine Product Formula.
From www.dreamstime.com
Elemental Bromine Br2, Molecule. Skeletal Formula Stock Vector Bromine Product Formula As an important chemical raw material, it is widely used in. The free bromine is then mixed with sulfur dioxide, and the. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Two products with multiple active ingredients and two products as the sole active ingredient. The product of the. Bromine Product Formula.
From www.alamy.com
Bromine molecule Br2 Stock Photo Alamy Bromine Product Formula It has an atomic weight of 79.904 and a mass. The image intends to reflect the rich colour, liquidity and aromatic nature of the element. Bromine is an active ingredient in four products; Bromine is mainly used to produce bromides. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35.. Bromine Product Formula.
From molekula.com
Purchase Bromine [7726956] online • Catalog • Molekula Group Bromine Product Formula Bromine is an active ingredient in four products; The multiple active ingredient products control. Bromine is mainly used to produce bromides. The product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. Bromine is the 35th element in the periodic table and has a symbol of br and atomic. Bromine Product Formula.
From solvedlib.com
Consider the reaction of bromine with the pictured al… SolvedLib Bromine Product Formula The image intends to reflect the rich colour, liquidity and aromatic nature of the element. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The multiple active ingredient products control. Bromine is mainly used to produce bromides. The free bromine is then mixed with sulfur dioxide, and the. Bromine. Bromine Product Formula.
From www.chegg.com
Solved Bromine and water react to form hydrogen bromide and Bromine Product Formula The image intends to reflect the rich colour, liquidity and aromatic nature of the element. The free bromine is then mixed with sulfur dioxide, and the. The multiple active ingredient products control. The product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. It has an atomic weight of. Bromine Product Formula.
From scienceinfo.com
Bromine (Br) Element Properties and Amazing Uses, Facts Bromine Product Formula It has an atomic weight of 79.904 and a mass. The image intends to reflect the rich colour, liquidity and aromatic nature of the element. The product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. As an important chemical raw material, it is widely used in. Bromine is. Bromine Product Formula.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Product Formula The image intends to reflect the rich colour, liquidity and aromatic nature of the element. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The free bromine is then mixed with sulfur dioxide, and the. It has an atomic weight of 79.904 and a mass. Two products with multiple. Bromine Product Formula.
From dawson-bogspotsheppard.blogspot.com
Bromine Water Test Equation Bromine Product Formula Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The free bromine is then mixed with sulfur dioxide, and the. The multiple active ingredient products control. As an important chemical raw material, it is widely used in. The product of the reaction is a dilute solution of bromine, from. Bromine Product Formula.
From www.pw.live
Bromine Formula, Valency, Mass And Properties Bromine Product Formula Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Bromine is an active ingredient in four products; The product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. Two products with multiple active ingredients and two products as. Bromine Product Formula.
From www.youtube.com
How to Balance Al + Br2 = AlBr3 (Aluminum + Bromine gas) YouTube Bromine Product Formula It has an atomic weight of 79.904 and a mass. Two products with multiple active ingredients and two products as the sole active ingredient. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. As an important chemical raw material, it is widely used in. The free bromine is then. Bromine Product Formula.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Product Formula It has an atomic weight of 79.904 and a mass. The product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. As an important chemical raw material, it is widely used in. Bromine is the 35th element in the periodic table and has a symbol of br and atomic. Bromine Product Formula.