Bromine Trioxide Anion . Recognize polyatomic ions in chemical formulas. Write the correct formula for an ionic compound. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. Dibromine trioxide is the chemical compound composed of bromine and oxygen with the formula br2o3. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. It is an orange solid that is. Bromine compounds are compounds containing the element bromine (br). Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine.
from www.semanticscholar.org
Dibromine trioxide is the chemical compound composed of bromine and oxygen with the formula br2o3. Bromine compounds are compounds containing the element bromine (br). Recognize polyatomic ions in chemical formulas. It is an orange solid that is. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. Write the correct formula for an ionic compound. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine.
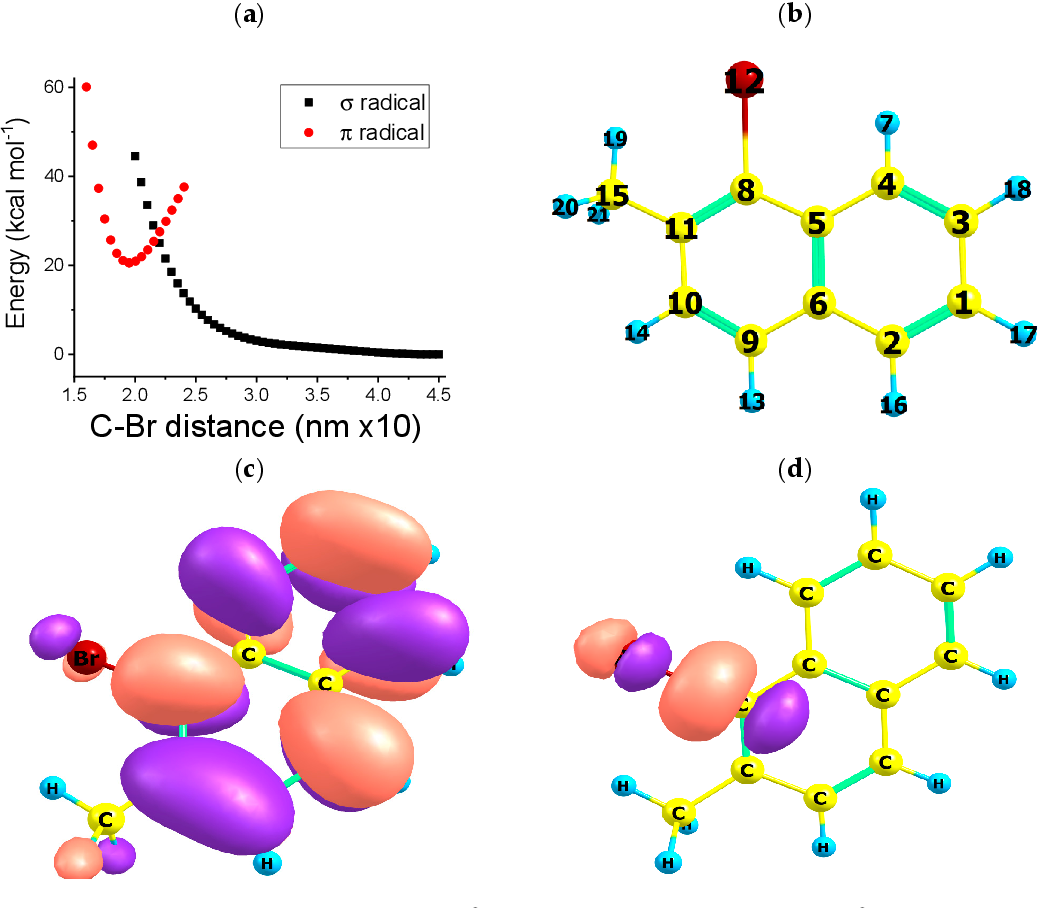
Figure 1 from On the Dynamics of the CarbonBromine Bond Dissociation
Bromine Trioxide Anion For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. It is an orange solid that is. Recognize polyatomic ions in chemical formulas. Write the correct formula for an ionic compound. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. Bromine compounds are compounds containing the element bromine (br). For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Dibromine trioxide is the chemical compound composed of bromine and oxygen with the formula br2o3.
From www.mdpi.com
Molecules Free FullText On the Dynamics of the CarbonBromine Bond Bromine Trioxide Anion Recognize polyatomic ions in chemical formulas. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. Write the correct formula for an ionic compound. Dibromine trioxide is the chemical compound composed of bromine and oxygen with the. Bromine Trioxide Anion.
From www.coursehero.com
[Solved] Neutral bromine (Br2) can gain two electrons to the Bromine Trioxide Anion Write the correct formula for an ionic compound. Bromine compounds are compounds containing the element bromine (br). Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. It is an orange solid that is. Dibromine trioxide is. Bromine Trioxide Anion.
From www.mdpi.com
Molecules Free FullText On the Dynamics of the CarbonBromine Bond Bromine Trioxide Anion It is an orange solid that is. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. Recognize polyatomic ions in chemical formulas. Bromine compounds are compounds containing the element bromine (br). Bond energies to bromine tend to be lower than those to chlorine but higher than those to. Bromine Trioxide Anion.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Trioxide Anion Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Dibromine trioxide is the chemical compound composed of bromine and oxygen with the formula br2o3. Bromine compounds. Bromine Trioxide Anion.
From www.semanticscholar.org
Figure 1 from On the Dynamics of the CarbonBromine Bond Dissociation Bromine Trioxide Anion Dibromine trioxide is the chemical compound composed of bromine and oxygen with the formula br2o3. It is an orange solid that is. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. Recognize polyatomic ions in chemical. Bromine Trioxide Anion.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Trioxide Anion Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Bond energies to bromine tend to be lower than those to chlorine but higher than those to. Bromine Trioxide Anion.
From www.chegg.com
Solved Draw a line connecting the chemical formula with the Bromine Trioxide Anion For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Bromine compounds are compounds containing the element bromine (br). Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a. Bromine Trioxide Anion.
From slideplayer.com
Atoms and Ions SNC2D. ppt download Bromine Trioxide Anion Dibromine trioxide is the chemical compound composed of bromine and oxygen with the formula br2o3. Bromine compounds are compounds containing the element bromine (br). Write the correct formula for an ionic compound. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. For example, the neutral bromine atom, with. Bromine Trioxide Anion.
From www.researchgate.net
Hydrogen bonds of two independent C5N2H14 2+ cations. Bromine olive Bromine Trioxide Anion Write the correct formula for an ionic compound. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one. Bromine Trioxide Anion.
From www.freepik.com
Premium Vector Bromine symbol Chemical element of the periodic table Bromine Trioxide Anion Write the correct formula for an ionic compound. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. Recognize polyatomic ions in chemical formulas. Bromine compounds are compounds containing the element bromine (br). Note, members of the. Bromine Trioxide Anion.
From www.vedantu.com
Revision Notes Class 11 Chemistry Ch 8 Redox Reactions Bromine Trioxide Anion Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. Recognize polyatomic ions in chemical formulas. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36. Bromine Trioxide Anion.
From www.coursehero.com
[Solved] Neutral bromine (Br2) can gain two electrons to the Bromine Trioxide Anion Write the correct formula for an ionic compound. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. It is an orange solid that is. Dibromine trioxide is the chemical compound composed of bromine and oxygen with the formula br2o3. Bromine compounds are compounds containing the element bromine (br).. Bromine Trioxide Anion.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine Trioxide Anion Dibromine trioxide is the chemical compound composed of bromine and oxygen with the formula br2o3. It is an orange solid that is. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. Recognize polyatomic ions in chemical. Bromine Trioxide Anion.
From www.numerade.com
SOLVED 1.Reaction of the bromide anion (Br) with elemental bromine Bromine Trioxide Anion Dibromine trioxide is the chemical compound composed of bromine and oxygen with the formula br2o3. It is an orange solid that is. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. Bromine compounds are compounds containing. Bromine Trioxide Anion.
From gioizlnut.blob.core.windows.net
Is Bromine Anion Or Cation at Marci Burrell blog Bromine Trioxide Anion Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. Write the correct formula for an ionic compound. Recognize polyatomic ions in chemical formulas. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. It is an. Bromine Trioxide Anion.
From www.youtube.com
Ionic Charge for Bromine (Br) YouTube Bromine Trioxide Anion Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. Recognize polyatomic ions in chemical formulas. Write the correct formula for an ionic compound. Dibromine trioxide is the chemical compound composed of bromine and oxygen with the. Bromine Trioxide Anion.
From slideplayer.com
Chemical Bonds. ppt download Bromine Trioxide Anion Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. Bromine compounds are compounds containing the element bromine (br). It is an orange solid that is. Recognize polyatomic ions in chemical formulas. Note, members of the same. Bromine Trioxide Anion.
From www.mdpi.com
Molecules Free FullText The Peroxymonocarbonate Anion HCO4− as an Bromine Trioxide Anion Write the correct formula for an ionic compound. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. Bromine compounds are compounds containing the element bromine (br). Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a. Bromine Trioxide Anion.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Trioxide Anion Recognize polyatomic ions in chemical formulas. Dibromine trioxide is the chemical compound composed of bromine and oxygen with the formula br2o3. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. It is an orange solid that. Bromine Trioxide Anion.
From www.numerade.com
SOLVEDWhich anion is labeled correctly? Select one a NO2 nitroxide b Bromine Trioxide Anion For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Recognize polyatomic ions in chemical formulas. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. It is an orange solid that is. Bond energies to bromine. Bromine Trioxide Anion.
From www.numerade.com
SOLVED Question 6 of 21 My Attempt Solution The methoxide anion will Bromine Trioxide Anion It is an orange solid that is. Write the correct formula for an ionic compound. Recognize polyatomic ions in chemical formulas. Dibromine trioxide is the chemical compound composed of bromine and oxygen with the formula br2o3. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Note, members. Bromine Trioxide Anion.
From www.chemistrystudent.com
Phenol Reactions (ALevel) ChemistryStudent Bromine Trioxide Anion Recognize polyatomic ions in chemical formulas. Bromine compounds are compounds containing the element bromine (br). Dibromine trioxide is the chemical compound composed of bromine and oxygen with the formula br2o3. It is an orange solid that is. Write the correct formula for an ionic compound. Note, members of the same family tend to form similar compounds, so bromine and iodine. Bromine Trioxide Anion.
From www.anyrgb.com
Bromine pentafluoride, xenon Oxytetrafluoride, bromine Trifluoride Bromine Trioxide Anion Bromine compounds are compounds containing the element bromine (br). For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a. Bromine Trioxide Anion.
From slideplayer.com
Unit 5 Ionic Bonding & Nomenclature ppt download Bromine Trioxide Anion Write the correct formula for an ionic compound. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. It is an orange solid that is. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker. Bromine Trioxide Anion.
From slideplayer.com
Review Review Cl1 Unstable Stable Stable ppt download Bromine Trioxide Anion Dibromine trioxide is the chemical compound composed of bromine and oxygen with the formula br2o3. It is an orange solid that is. Bromine compounds are compounds containing the element bromine (br). Recognize polyatomic ions in chemical formulas. Write the correct formula for an ionic compound. Bond energies to bromine tend to be lower than those to chlorine but higher than. Bromine Trioxide Anion.
From sci-toys.com
bromine trioxide Bromine Trioxide Anion Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. Recognize polyatomic ions in chemical formulas.. Bromine Trioxide Anion.
From openpress.usask.ca
12.7. Oxidation of Alcohols via Elimination Introduction to Organic Bromine Trioxide Anion Dibromine trioxide is the chemical compound composed of bromine and oxygen with the formula br2o3. Write the correct formula for an ionic compound. Bromine compounds are compounds containing the element bromine (br). Recognize polyatomic ions in chemical formulas. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. Bond. Bromine Trioxide Anion.
From www.researchgate.net
(PDF) Bromine‐Induced Defects in Anion‐Deficient Zinc Oxide as Stable Bromine Trioxide Anion Dibromine trioxide is the chemical compound composed of bromine and oxygen with the formula br2o3. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. It is an orange solid that is. Note, members of the same family tend to form similar compounds, so bromine and iodine form. Bromine Trioxide Anion.
From www.doubtnut.com
Consider the change in oxidation state of Bromine corredponding to dif Bromine Trioxide Anion Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Dibromine trioxide is the chemical. Bromine Trioxide Anion.
From h-o-m-e.org
Decoding The Ionic Charge of Bromine Bromine Trioxide Anion It is an orange solid that is. Dibromine trioxide is the chemical compound composed of bromine and oxygen with the formula br2o3. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Note, members of the same family tend to form similar compounds, so bromine and iodine form. Bromine Trioxide Anion.
From slidetodoc.com
Sn F 2 FORMULA TIN II FLUORIDE Plumbic Bromine Trioxide Anion Recognize polyatomic ions in chemical formulas. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. Dibromine trioxide is the chemical compound composed of bromine and oxygen with the formula br2o3. Write the correct formula for an ionic compound. Bond energies to bromine tend to be lower than those. Bromine Trioxide Anion.
From www.researchgate.net
Arrangement of four C5N2H14 2+ cations around the Br − anion. Bromine Bromine Trioxide Anion Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. Dibromine trioxide is the chemical compound composed of bromine and oxygen with the formula br2o3. It is an orange solid that is. Bond energies to bromine tend to be lower than those to chlorine but higher than those to. Bromine Trioxide Anion.
From cdnsciencepub.com
A facile nucleophilic displacement of bromine in 2amino4methyl5 Bromine Trioxide Anion Dibromine trioxide is the chemical compound composed of bromine and oxygen with the formula br2o3. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. Note, members of the same family tend to form similar compounds, so. Bromine Trioxide Anion.
From www.researchgate.net
Structure of [Pt(η 2P 3 )(η 2tripos)] + [14]. Six bromine atoms form Bromine Trioxide Anion Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. Write the correct formula for an ionic compound. It is an orange solid that is. Dibromine trioxide is the chemical compound composed of bromine and oxygen with the formula br2o3. Bromine compounds are compounds containing the element bromine (br).. Bromine Trioxide Anion.
From www.youtube.com
Electron Configuration of Bromine II Easy & Quick YouTube Bromine Trioxide Anion Recognize polyatomic ions in chemical formulas. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Dibromine trioxide is the chemical compound composed of bromine and oxygen. Bromine Trioxide Anion.