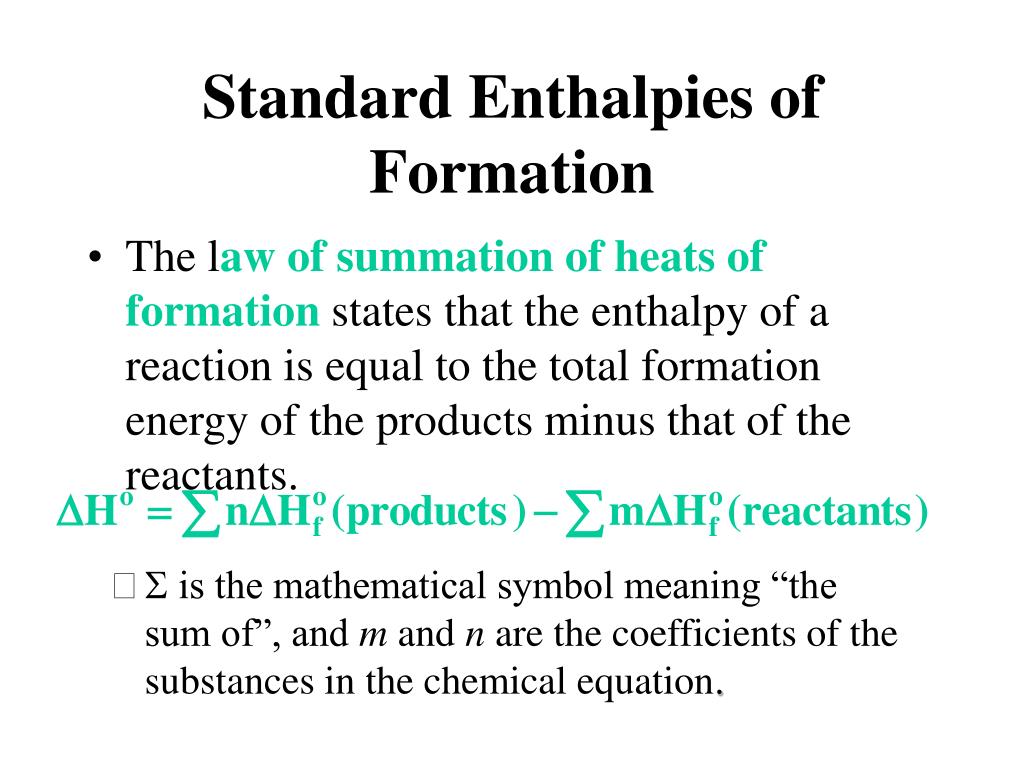
Standard Enthalpy Of Formation Units . 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in. for any substance at any particular temperature, we define the standard enthalpy of formation as the. Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances. a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its stoichiometric coefficient) minus the sum of the standard enthalpies of the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a.
from www.slideserve.com
Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its stoichiometric coefficient) minus the sum of the standard enthalpies of the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in. for any substance at any particular temperature, we define the standard enthalpy of formation as the. a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a.
PPT Standard Enthalpies of Formation PowerPoint Presentation, free
Standard Enthalpy Of Formation Units the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. for any substance at any particular temperature, we define the standard enthalpy of formation as the. a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its stoichiometric coefficient) minus the sum of the standard enthalpies of the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances. Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in.
From www.thesciencehive.co.uk
Enthalpy Changes — the science hive Standard Enthalpy Of Formation Units the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. for any substance at any particular temperature, we define the standard enthalpy of formation as the. the standard enthalpy of formation is a measure. Standard Enthalpy Of Formation Units.
From stahonorschemistry.weebly.com
III Calculating Enthalpies STA Form IV Honors Chemistry Standard Enthalpy Of Formation Units the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its stoichiometric coefficient) minus the sum of the standard enthalpies of the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance. Standard Enthalpy Of Formation Units.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Units a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change. Standard Enthalpy Of Formation Units.
From www.chegg.com
Solved Use The Standard Enthalpies Of Formation In The Ta... Standard Enthalpy Of Formation Units Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in.. Standard Enthalpy Of Formation Units.
From www.chegg.com
Solved Using the table of standard enthalpies of formation Standard Enthalpy Of Formation Units for any substance at any particular temperature, we define the standard enthalpy of formation as the. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in. Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to. the standard. Standard Enthalpy Of Formation Units.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Units the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of. Standard Enthalpy Of Formation Units.
From mavink.com
Enthalpy Of Formation Equation Standard Enthalpy Of Formation Units the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its stoichiometric coefficient) minus the sum of the standard enthalpies of a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a. the. Standard Enthalpy Of Formation Units.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in Standard Enthalpy Of Formation Units the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its stoichiometric coefficient) minus the sum of the standard enthalpies of the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance. Standard Enthalpy Of Formation Units.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation Standard Enthalpy Of Formation Units 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in. a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change. Standard Enthalpy Of Formation Units.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation Units the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. for any substance at any particular temperature, we define the standard enthalpy of formation as the. Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to. 193 rows the. Standard Enthalpy Of Formation Units.
From www.slideserve.com
PPT Formation Reactions PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Units the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. Use standard enthalpies of formation to. Standard Enthalpy Of Formation Units.
From www.youtube.com
How to Calculate the Standard Enthalpy of a Reaction from the Standard Standard Enthalpy Of Formation Units the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its stoichiometric coefficient) minus the sum of the standard enthalpies of 193 rows the. Standard Enthalpy Of Formation Units.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Units a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when. Standard Enthalpy Of Formation Units.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Units 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in. the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole. Standard Enthalpy Of Formation Units.
From www.chegg.com
Solved 13. The standard enthalpies of formation for several Standard Enthalpy Of Formation Units the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in. for any substance at. Standard Enthalpy Of Formation Units.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Of Formation Units the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances. a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of. Standard Enthalpy Of Formation Units.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Of Formation Units the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its. Standard Enthalpy Of Formation Units.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Units 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in. Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole. Standard Enthalpy Of Formation Units.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Units the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances. the standard enthalpy of formation is. Standard Enthalpy Of Formation Units.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Units the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. a standard enthalpy of formation $δh. Standard Enthalpy Of Formation Units.
From www.nagwa.com
Question Video Calculating the Standard Enthalpy of Formation for Standard Enthalpy Of Formation Units a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies. Standard Enthalpy Of Formation Units.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Units the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances. a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of. Standard Enthalpy Of Formation Units.
From www.studocu.com
Standard Enthalpies of Formation & Standard Entropies kJ J ( mol Standard Enthalpy Of Formation Units the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a. for any substance. Standard Enthalpy Of Formation Units.
From www.numerade.com
SOLVED 'The following problems deal with standard enthalpy of Standard Enthalpy Of Formation Units for any substance at any particular temperature, we define the standard enthalpy of formation as the. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to. the standard enthalpy. Standard Enthalpy Of Formation Units.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation Units the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to. the standard enthalpy of formation is. Standard Enthalpy Of Formation Units.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Units Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to. for any substance at any particular temperature, we define the standard enthalpy of formation as the. the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances. 193 rows the standard. Standard Enthalpy Of Formation Units.
From thechemistrynotes.com
Enthalpy Introduction, Calculation, Enthalpy change, Importance Standard Enthalpy Of Formation Units the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. for any substance at any particular temperature, we define the standard enthalpy of formation as the. the standard enthalpy of formation is a measure. Standard Enthalpy Of Formation Units.
From schoolworkhelper.net
Standard Enthalpies of Formation Online Homework Help SchoolWorkHelper Standard Enthalpy Of Formation Units 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. Use standard enthalpies of formation. Standard Enthalpy Of Formation Units.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Units the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances. for any substance at any particular temperature, we define the standard enthalpy of formation as the. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in. Use standard enthalpies. Standard Enthalpy Of Formation Units.
From www.slideserve.com
PPT Standard Enthalpy Changes = D H o PowerPoint Presentation, free Standard Enthalpy Of Formation Units for any substance at any particular temperature, we define the standard enthalpy of formation as the. a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a. the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances. the. Standard Enthalpy Of Formation Units.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Units Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of. Standard Enthalpy Of Formation Units.
From www.toppr.com
Use the given standard enthalpies of formation (in kJ/mol) to determine Standard Enthalpy Of Formation Units the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in. Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to. . Standard Enthalpy Of Formation Units.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Units 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in. for any substance at any particular temperature, we define the standard enthalpy of formation as the. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products. Standard Enthalpy Of Formation Units.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF Standard Enthalpy Of Formation Units 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in. Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a.. Standard Enthalpy Of Formation Units.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work Standard Enthalpy Of Formation Units for any substance at any particular temperature, we define the standard enthalpy of formation as the. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each. Standard Enthalpy Of Formation Units.