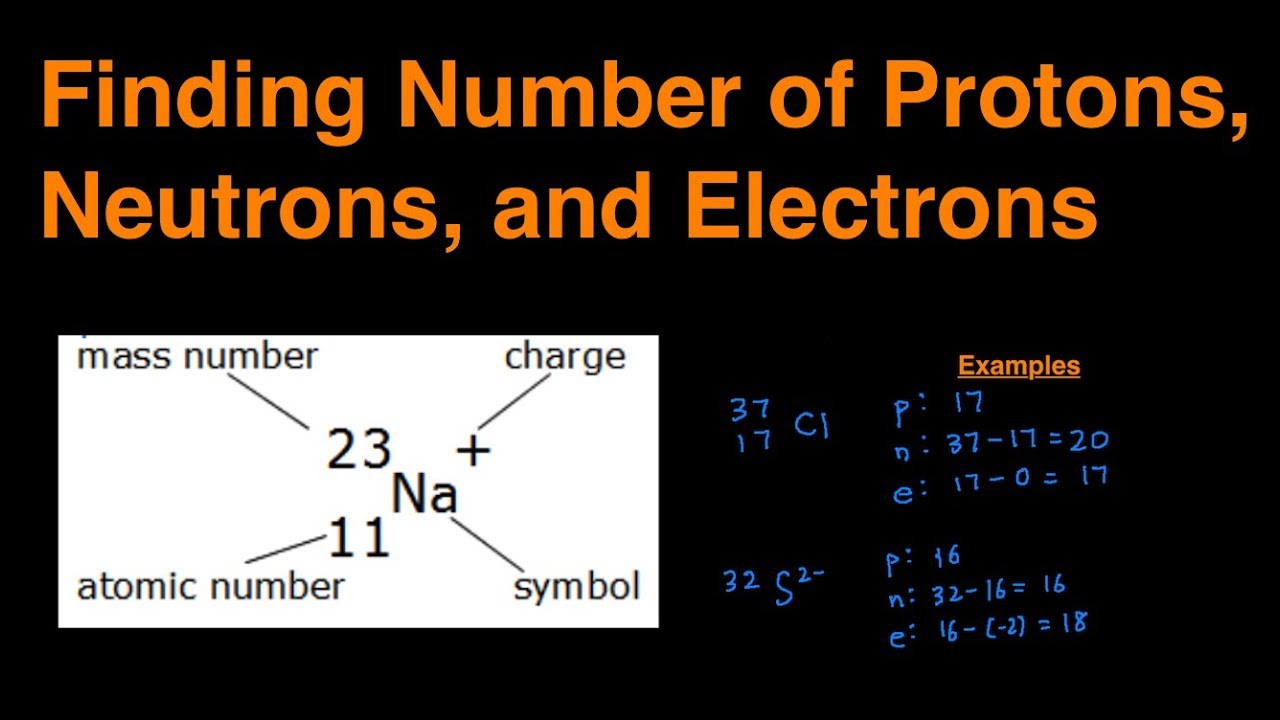
How Do U Work Out How Many Electrons . The electronic structure of an atom can be predicted from the atom's. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. Determine the number of protons and electrons. For example, the atomic number of sodium is 11. In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons, neutrons, and electrons in an atom or element Describe the locations, charges, and masses of the three main subatomic particles. For example, the atomic number of sodium is 11. We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. The electronic structure of an atom can be predicted from its atomic number.
from www.youtube.com
Determine the number of protons and electrons. The electronic structure of an atom can be predicted from its atomic number. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. For example, the atomic number of sodium is 11. For example, the atomic number of sodium is 11. The electronic structure of an atom can be predicted from the atom's. In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons, neutrons, and electrons in an atom or element We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: Describe the locations, charges, and masses of the three main subatomic particles.
How to Determine Number of Protons, Neutrons, and Electrons. Step by
How Do U Work Out How Many Electrons Describe the locations, charges, and masses of the three main subatomic particles. For example, the atomic number of sodium is 11. For example, the atomic number of sodium is 11. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. The electronic structure of an atom can be predicted from the atom's. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. The electronic structure of an atom can be predicted from its atomic number. We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons, neutrons, and electrons in an atom or element Determine the number of protons and electrons. Describe the locations, charges, and masses of the three main subatomic particles.
From www.animalia-life.club
Electrons In An Atom How Do U Work Out How Many Electrons This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. Describe the locations, charges, and masses of the three main subatomic particles. The electronic structure of an atom can be predicted from its atomic number. We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of. How Do U Work Out How Many Electrons.
From alevelchemistry.co.uk
Electron Structure ALevel Chemistry Revision Notes How Do U Work Out How Many Electrons The electronic structure of an atom can be predicted from its atomic number. For example, the atomic number of sodium is 11. Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons and electrons. The electronic structure of an atom can be predicted from the atom's. You can use these numbers to calculate. How Do U Work Out How Many Electrons.
From utedzz.blogspot.com
Valence Electrons Periodic Table Transition Metals Periodic Table How Do U Work Out How Many Electrons The electronic structure of an atom can be predicted from its atomic number. We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: Describe the locations, charges, and masses of the three main subatomic particles. You can use these numbers to calculate the number of. How Do U Work Out How Many Electrons.
From quizzlibmisty.z21.web.core.windows.net
How To Find The Protons Neutrons Electrons How Do U Work Out How Many Electrons Determine the number of protons and electrons. For example, the atomic number of sodium is 11. The electronic structure of an atom can be predicted from the atom's. The electronic structure of an atom can be predicted from its atomic number. For example, the atomic number of sodium is 11. We know that the mass number (a) = number of. How Do U Work Out How Many Electrons.
From www.nagwa.com
Question Video Identifying the Number of Electrons in the Outermost How Do U Work Out How Many Electrons For example, the atomic number of sodium is 11. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. The electronic structure of an atom can be predicted from the atom's. For example, the atomic number of sodium is 11. Determine the number of protons and electrons. In this chemtalk tutorial, you will learn how to. How Do U Work Out How Many Electrons.
From www.wikihow.com
How to Find Electrons 6 Steps (with Pictures) wikiHow How Do U Work Out How Many Electrons For example, the atomic number of sodium is 11. Determine the number of protons and electrons. For example, the atomic number of sodium is 11. The electronic structure of an atom can be predicted from its atomic number. In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons, neutrons, and electrons in an. How Do U Work Out How Many Electrons.
From www.youtube.com
How to find the number of Protons, Neutrons and Electrons? Chemistry How Do U Work Out How Many Electrons The electronic structure of an atom can be predicted from the atom's. For example, the atomic number of sodium is 11. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. For example, the atomic number of sodium is 11. Determine the number of protons and electrons. The electronic structure of an atom. How Do U Work Out How Many Electrons.
From www.sliderbase.com
Atoms and the Periodic table Presentation Chemistry How Do U Work Out How Many Electrons The electronic structure of an atom can be predicted from its atomic number. We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: Determine the number of protons and electrons. For example, the atomic number of sodium is 11. In this chemtalk tutorial, you will. How Do U Work Out How Many Electrons.
From www.slideserve.com
PPT How are electrons arranged? PowerPoint Presentation, free How Do U Work Out How Many Electrons Describe the locations, charges, and masses of the three main subatomic particles. We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: The electronic structure of an atom can be predicted from the atom's. In this chemtalk tutorial, you will learn how to easily calculate. How Do U Work Out How Many Electrons.
From www.britannica.com
Electron Definition, Mass, & Facts Britannica How Do U Work Out How Many Electrons For example, the atomic number of sodium is 11. We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. The electronic structure of an atom can be predicted from its atomic. How Do U Work Out How Many Electrons.
From www.wikihow.com
How to Find the Number of Neutrons in an Atom 11 Steps How Do U Work Out How Many Electrons Determine the number of protons and electrons. For example, the atomic number of sodium is 11. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: The electronic. How Do U Work Out How Many Electrons.
From www.youtube.com
Physics How many electrons are in 1 coulomb? YouTube How Do U Work Out How Many Electrons Determine the number of protons and electrons. In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons, neutrons, and electrons in an atom or element The electronic structure of an atom can be predicted from the atom's. You can use these numbers to calculate the number of protons, neutrons and electrons in an. How Do U Work Out How Many Electrons.
From sciencenotes.org
List of Electron Configurations of Elements How Do U Work Out How Many Electrons You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. For example, the atomic number of sodium is 11. In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons, neutrons, and electrons. How Do U Work Out How Many Electrons.
From www.youtube.com
HOW TO FIND NUMBER OF NEUTRONS, ELECTRONS AND PROTONS YouTube How Do U Work Out How Many Electrons For example, the atomic number of sodium is 11. Determine the number of protons and electrons. Describe the locations, charges, and masses of the three main subatomic particles. We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: For example, the atomic number of sodium. How Do U Work Out How Many Electrons.
From www.wikihow.com
How to Find Electrons 7 Steps (with Pictures) wikiHow How Do U Work Out How Many Electrons Determine the number of protons and electrons. The electronic structure of an atom can be predicted from its atomic number. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons, neutrons, and electrons in an atom or element You. How Do U Work Out How Many Electrons.
From www.youtube.com
Determining the number of electrons YouTube How Do U Work Out How Many Electrons Determine the number of protons and electrons. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. The electronic structure of an atom can be predicted from its atomic number. We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: For. How Do U Work Out How Many Electrons.
From www.wikihow.com
How to Find Electrons 7 Steps (with Pictures) wikiHow How Do U Work Out How Many Electrons In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons, neutrons, and electrons in an atom or element For example, the atomic number of sodium is 11. For example, the atomic number of sodium is 11. The electronic structure of an atom can be predicted from the atom's. You can use these numbers. How Do U Work Out How Many Electrons.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID5413991 How Do U Work Out How Many Electrons Determine the number of protons and electrons. For example, the atomic number of sodium is 11. For example, the atomic number of sodium is 11. In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons, neutrons, and electrons in an atom or element This chemistry video tutorial explains how to calculate the number. How Do U Work Out How Many Electrons.
From icdsc.org
How many electrons are in each shell including 3p orbitals How Do U Work Out How Many Electrons Determine the number of protons and electrons. For example, the atomic number of sodium is 11. The electronic structure of an atom can be predicted from its atomic number. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. Describe the locations, charges, and masses of the three main subatomic particles. In this. How Do U Work Out How Many Electrons.
From www.wikihow.com
How to Find Electrons 7 Steps (with Pictures) wikiHow How Do U Work Out How Many Electrons For example, the atomic number of sodium is 11. Determine the number of protons and electrons. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. Describe the locations, charges, and masses of the three main subatomic particles. For example, the atomic number of sodium is 11. We know that the mass number (a) = number. How Do U Work Out How Many Electrons.
From www.youtube.com
How To Calculate The Number of Protons, Neutrons, and Electrons How Do U Work Out How Many Electrons Describe the locations, charges, and masses of the three main subatomic particles. In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons, neutrons, and electrons in an atom or element You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. For example, the atomic number of. How Do U Work Out How Many Electrons.
From www.slideserve.com
PPT How many protons, neutrons & electrons are present in atom How Do U Work Out How Many Electrons The electronic structure of an atom can be predicted from the atom's. We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: For example, the atomic number of sodium is 11. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and.. How Do U Work Out How Many Electrons.
From www.youtube.com
How to work out the electron configuration of an element YouTube How Do U Work Out How Many Electrons In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons, neutrons, and electrons in an atom or element For example, the atomic number of sodium is 11. The electronic structure of an atom can be predicted from the atom's. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and.. How Do U Work Out How Many Electrons.
From slideplayer.com
Structure of the Atom & The Periodic Table. ppt download How Do U Work Out How Many Electrons The electronic structure of an atom can be predicted from its atomic number. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons, neutrons, and electrons in an atom or element Describe the locations, charges, and masses of the. How Do U Work Out How Many Electrons.
From www.slideserve.com
PPT Protons and Electrons PowerPoint Presentation, free download ID How Do U Work Out How Many Electrons The electronic structure of an atom can be predicted from the atom's. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. The electronic structure of an atom can be predicted from its atomic number. Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons and electrons. You can. How Do U Work Out How Many Electrons.
From www.youtube.com
How to Determine Number of Protons, Neutrons, and Electrons. Step by How Do U Work Out How Many Electrons You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. The electronic structure of an atom can be predicted from its atomic number. Describe the locations, charges, and masses of the three main subatomic particles. For example, the atomic number of sodium is 11. This chemistry video tutorial explains how to calculate the. How Do U Work Out How Many Electrons.
From www.slideserve.com
PPT Properties of Atoms PowerPoint Presentation, free download ID How Do U Work Out How Many Electrons Describe the locations, charges, and masses of the three main subatomic particles. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. The electronic structure of an atom can be predicted from the atom's. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. In this chemtalk tutorial, you. How Do U Work Out How Many Electrons.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho How Do U Work Out How Many Electrons For example, the atomic number of sodium is 11. Determine the number of protons and electrons. The electronic structure of an atom can be predicted from its atomic number. For example, the atomic number of sodium is 11. We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons. How Do U Work Out How Many Electrons.
From www.slideserve.com
PPT Quantum Number and Electron Configurations PowerPoint How Do U Work Out How Many Electrons You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. The electronic structure of an atom can be predicted from the atom's. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. For example, the atomic number of sodium is 11. Describe the locations, charges, and masses of the. How Do U Work Out How Many Electrons.
From icdsc.org
How many electrons are in each shell including 3p orbitals How Do U Work Out How Many Electrons The electronic structure of an atom can be predicted from its atomic number. Determine the number of protons and electrons. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: You. How Do U Work Out How Many Electrons.
From www.expii.com
Electrons — Structure & Properties Expii How Do U Work Out How Many Electrons You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. For example, the atomic number of sodium is 11. For example, the atomic number of sodium is 11. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. Determine the number of protons and electrons. The electronic structure of. How Do U Work Out How Many Electrons.
From quizzlibmisty.z21.web.core.windows.net
How To Find The Protons Neutrons Electrons How Do U Work Out How Many Electrons We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: The electronic structure of an atom can be predicted from its atomic number. For example, the atomic number of sodium is 11. Describe the locations, charges, and masses of the three main subatomic particles. This. How Do U Work Out How Many Electrons.
From www.nagwa.com
Question Video Identifying the Number of Electrons in Shells Nagwa How Do U Work Out How Many Electrons In this chemtalk tutorial, you will learn how to easily calculate and find the number or protons, neutrons, and electrons in an atom or element This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. For example, the atomic number of sodium is 11. The electronic structure of an atom can be predicted from its atomic. How Do U Work Out How Many Electrons.
From www.sliderbase.com
How many protons, electrons, and neutrons are in an atom Presentation How Do U Work Out How Many Electrons You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons and electrons. For example, the atomic number of sodium is 11. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and. The. How Do U Work Out How Many Electrons.
From www.wikihow.com
How to Find the Number of Protons, Neutrons, and Electrons How Do U Work Out How Many Electrons You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. We know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: For example, the atomic number of sodium is 11. This chemistry video tutorial explains how to calculate the. How Do U Work Out How Many Electrons.