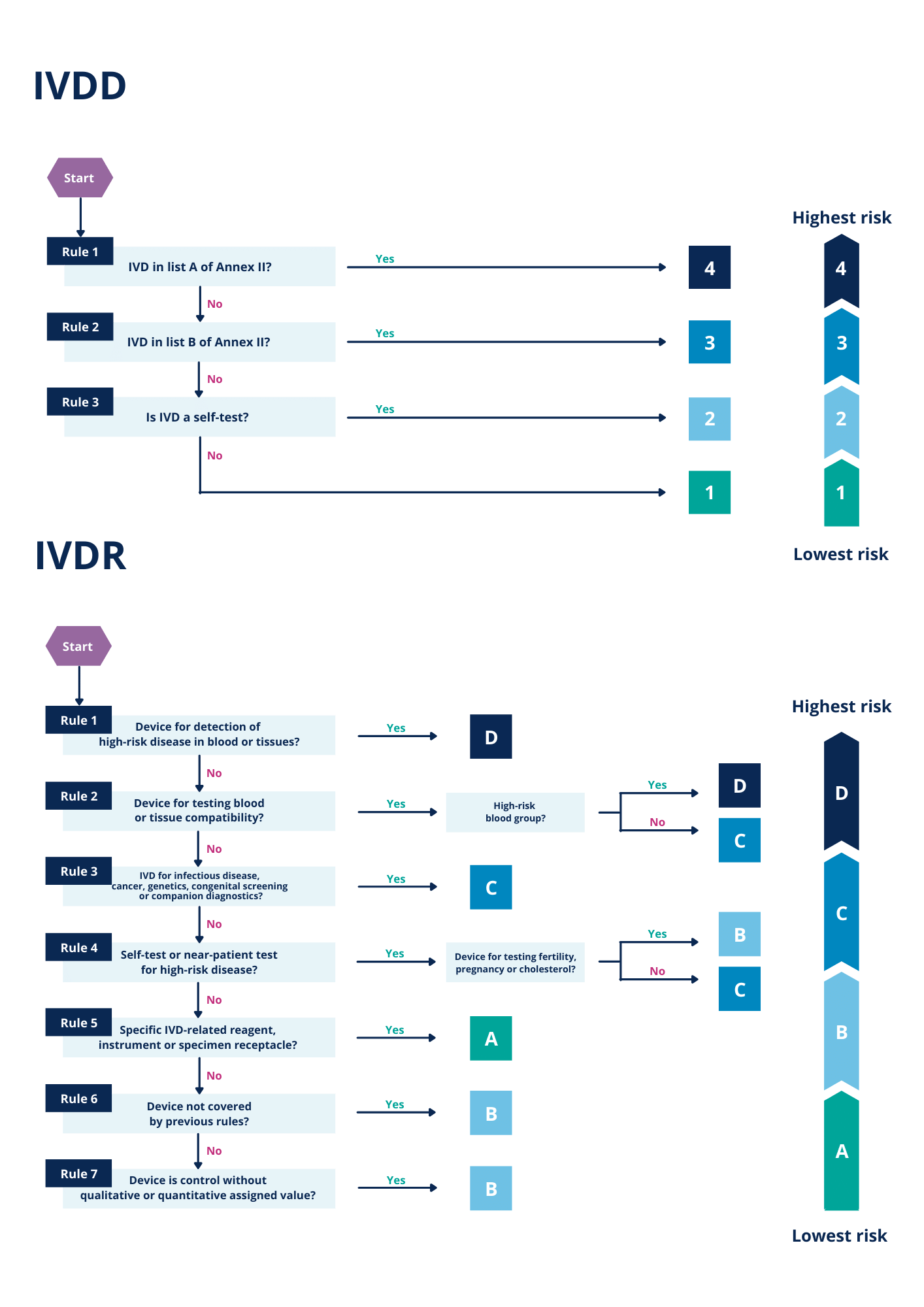
Principles Of In Vitro Diagnostic Medical Devices Classification . Its purpose is to assist a. “‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit,. This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) addresses the. This guidance document is one of a series that together describe a global regulatory model for medical devices. Principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) This document describes the principles of ivd medical devices classification in accordance with the requirements of the medical device. The new ivdr aligns the regulatory approach for ivd medical devices with that of the medical devices in general by establishing a set of risk.
from qbdgroup.com
This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) addresses the. “‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit,. Its purpose is to assist a. The new ivdr aligns the regulatory approach for ivd medical devices with that of the medical devices in general by establishing a set of risk. This guidance document is one of a series that together describe a global regulatory model for medical devices. This document describes the principles of ivd medical devices classification in accordance with the requirements of the medical device. Principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb)
IVDR classification of invitro diagnostic medical devices a brief guide
Principles Of In Vitro Diagnostic Medical Devices Classification This guidance document is one of a series that together describe a global regulatory model for medical devices. This document describes the principles of ivd medical devices classification in accordance with the requirements of the medical device. This guidance document is one of a series that together describe a global regulatory model for medical devices. This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) addresses the. The new ivdr aligns the regulatory approach for ivd medical devices with that of the medical devices in general by establishing a set of risk. “‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit,. Principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) Its purpose is to assist a.
From www.tuvsud.cn
Infographic The In Vitro Diagnostic Medical Device Regulation TÜV南德 Principles Of In Vitro Diagnostic Medical Devices Classification This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) addresses the. This guidance document is one of a series that together describe a global regulatory model for medical devices. Principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) “‘in vitro diagnostic medical device’ means any medical device which is. Principles Of In Vitro Diagnostic Medical Devices Classification.
From es.linkedin.com
News from IMDRF ‘Principles of In Vitro Diagnostic (IVD) Medical Principles Of In Vitro Diagnostic Medical Devices Classification Its purpose is to assist a. This guidance document is one of a series that together describe a global regulatory model for medical devices. The new ivdr aligns the regulatory approach for ivd medical devices with that of the medical devices in general by establishing a set of risk. “‘in vitro diagnostic medical device’ means any medical device which is. Principles Of In Vitro Diagnostic Medical Devices Classification.
From www.clinicalresearchassociatecra.com
Clinical Trial Basics on In Vitro Diagnostic Development (IVD Principles Of In Vitro Diagnostic Medical Devices Classification This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) addresses the. Its purpose is to assist a. The new ivdr aligns the regulatory approach for ivd medical devices with that of the medical devices in general by establishing a set of risk. This guidance document is one of a series that together. Principles Of In Vitro Diagnostic Medical Devices Classification.
From www.thermofisher.cn
IVDD vs. IVDR Classifications Defined and Compared OEMpowered Principles Of In Vitro Diagnostic Medical Devices Classification This guidance document is one of a series that together describe a global regulatory model for medical devices. “‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit,. Principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) Its purpose is to assist a. This document describes the principles. Principles Of In Vitro Diagnostic Medical Devices Classification.
From www.researchgate.net
Overview of the process of in vitro diagnostic (IVD) test development Principles Of In Vitro Diagnostic Medical Devices Classification The new ivdr aligns the regulatory approach for ivd medical devices with that of the medical devices in general by establishing a set of risk. Its purpose is to assist a. Principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) “‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control. Principles Of In Vitro Diagnostic Medical Devices Classification.
From www.academia.edu
(PDF) A Review on global harmonization task force (GHTF) principles Principles Of In Vitro Diagnostic Medical Devices Classification This document describes the principles of ivd medical devices classification in accordance with the requirements of the medical device. This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) addresses the. Its purpose is to assist a. “‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator,. Principles Of In Vitro Diagnostic Medical Devices Classification.
From operonstrategist.com
Guide to In Vitro Diagnostic Medical Device Regulation (IVDR) IVD Principles Of In Vitro Diagnostic Medical Devices Classification This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) addresses the. Its purpose is to assist a. “‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit,. The new ivdr aligns the regulatory approach for ivd medical devices with that of the medical. Principles Of In Vitro Diagnostic Medical Devices Classification.
From apacmed.org
What Is In Vitro Diagnostics (IVD) Types, Benefits & Regulations Principles Of In Vitro Diagnostic Medical Devices Classification The new ivdr aligns the regulatory approach for ivd medical devices with that of the medical devices in general by establishing a set of risk. “‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit,. This guidance document is one of a series that together describe a global regulatory model for. Principles Of In Vitro Diagnostic Medical Devices Classification.
From www.slideserve.com
PPT IVD and Point of care testing PowerPoint Presentation ID6646789 Principles Of In Vitro Diagnostic Medical Devices Classification This guidance document is one of a series that together describe a global regulatory model for medical devices. Its purpose is to assist a. Principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) “‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit,. This document describes the principles. Principles Of In Vitro Diagnostic Medical Devices Classification.
From www.slideshare.net
Regulation of In Vitro Diagnostic Medical Devices Transition to the… Principles Of In Vitro Diagnostic Medical Devices Classification This document describes the principles of ivd medical devices classification in accordance with the requirements of the medical device. Principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) “‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit,. Its purpose is to assist a. This guidance, relating to. Principles Of In Vitro Diagnostic Medical Devices Classification.
From www.mdpi.com
A Systematic Database Approach to Identify Companion Diagnostic Testing Principles Of In Vitro Diagnostic Medical Devices Classification This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) addresses the. The new ivdr aligns the regulatory approach for ivd medical devices with that of the medical devices in general by establishing a set of risk. This document describes the principles of ivd medical devices classification in accordance with the requirements of. Principles Of In Vitro Diagnostic Medical Devices Classification.
From operonstrategist.com
(IVDR) IN Vitro Diagnostic Medical Device Regulation IVDR Explained Principles Of In Vitro Diagnostic Medical Devices Classification The new ivdr aligns the regulatory approach for ivd medical devices with that of the medical devices in general by establishing a set of risk. Principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) addresses the. “‘in vitro diagnostic medical. Principles Of In Vitro Diagnostic Medical Devices Classification.
From studylib.net
Principles of In Vitro Diagnostic International Medical Device Principles Of In Vitro Diagnostic Medical Devices Classification This document describes the principles of ivd medical devices classification in accordance with the requirements of the medical device. “‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit,. This guidance document is one of a series that together describe a global regulatory model for medical devices. Its purpose is to. Principles Of In Vitro Diagnostic Medical Devices Classification.
From www.scilife.io
In Vitro Diagnostics (IVD) A Complete Overview Scilife Principles Of In Vitro Diagnostic Medical Devices Classification Its purpose is to assist a. This guidance document is one of a series that together describe a global regulatory model for medical devices. Principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) The new ivdr aligns the regulatory approach for ivd medical devices with that of the medical devices in general by establishing a set of. Principles Of In Vitro Diagnostic Medical Devices Classification.
From www.imdrf.org
Principles of In Vitro Diagnostic (IVD) Medical Devices Classification Principles Of In Vitro Diagnostic Medical Devices Classification The new ivdr aligns the regulatory approach for ivd medical devices with that of the medical devices in general by establishing a set of risk. This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) addresses the. This guidance document is one of a series that together describe a global regulatory model for. Principles Of In Vitro Diagnostic Medical Devices Classification.
From qbdgroup.com
IVDR classification of invitro diagnostic medical devices a brief guide Principles Of In Vitro Diagnostic Medical Devices Classification This guidance document is one of a series that together describe a global regulatory model for medical devices. The new ivdr aligns the regulatory approach for ivd medical devices with that of the medical devices in general by establishing a set of risk. Its purpose is to assist a. “‘in vitro diagnostic medical device’ means any medical device which is. Principles Of In Vitro Diagnostic Medical Devices Classification.
From gsap.co.il
In Vitro Diagnostic Medical Device (IVD) in the EU Gsap Principles Of In Vitro Diagnostic Medical Devices Classification The new ivdr aligns the regulatory approach for ivd medical devices with that of the medical devices in general by establishing a set of risk. “‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit,. Its purpose is to assist a. This guidance document is one of a series that together. Principles Of In Vitro Diagnostic Medical Devices Classification.
From www.slideserve.com
PPT Parameters For Classification Of Medical And InVitro Diagnostic Principles Of In Vitro Diagnostic Medical Devices Classification This document describes the principles of ivd medical devices classification in accordance with the requirements of the medical device. The new ivdr aligns the regulatory approach for ivd medical devices with that of the medical devices in general by establishing a set of risk. Its purpose is to assist a. This guidance, relating to the application of regulation (eu) 2017/746. Principles Of In Vitro Diagnostic Medical Devices Classification.
From www.slideserve.com
PPT Parameters For Classification Of Medical And InVitro Diagnostic Principles Of In Vitro Diagnostic Medical Devices Classification “‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit,. This document describes the principles of ivd medical devices classification in accordance with the requirements of the medical device. This guidance document is one of a series that together describe a global regulatory model for medical devices. Its purpose is to. Principles Of In Vitro Diagnostic Medical Devices Classification.
From www.arc-regulatory.com
InVitro Diagnostics (IVD) & Medical Device Expertise Consulting ARC Principles Of In Vitro Diagnostic Medical Devices Classification “‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit,. This guidance document is one of a series that together describe a global regulatory model for medical devices. The new ivdr aligns the regulatory approach for ivd medical devices with that of the medical devices in general by establishing a set. Principles Of In Vitro Diagnostic Medical Devices Classification.
From laegemiddelstyrelsen.dk
Classification of in vitro diagnostic medical devices (IVD) Principles Of In Vitro Diagnostic Medical Devices Classification The new ivdr aligns the regulatory approach for ivd medical devices with that of the medical devices in general by establishing a set of risk. Principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) “‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit,. Its purpose is to. Principles Of In Vitro Diagnostic Medical Devices Classification.
From en.icmcert.com
ICMC (International Certification Management Center Co., Ltd.) In Vitro Principles Of In Vitro Diagnostic Medical Devices Classification This document describes the principles of ivd medical devices classification in accordance with the requirements of the medical device. Principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) addresses the. The new ivdr aligns the regulatory approach for ivd medical. Principles Of In Vitro Diagnostic Medical Devices Classification.
From dokumen.tips
(PDF) Principles of In Vitro Diagnostic (IVD) Medical Devices Principles Of In Vitro Diagnostic Medical Devices Classification This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) addresses the. The new ivdr aligns the regulatory approach for ivd medical devices with that of the medical devices in general by establishing a set of risk. Its purpose is to assist a. “‘in vitro diagnostic medical device’ means any medical device which. Principles Of In Vitro Diagnostic Medical Devices Classification.
From www.medicalmicromolding.com
UK Medical Device Classification Principles Of In Vitro Diagnostic Medical Devices Classification Its purpose is to assist a. Principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) This guidance document is one of a series that together describe a global regulatory model for medical devices. This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) addresses the. This document describes the principles. Principles Of In Vitro Diagnostic Medical Devices Classification.
From qbdgroup.com
IVDR classification of invitro diagnostic medical devices a brief guide Principles Of In Vitro Diagnostic Medical Devices Classification This document describes the principles of ivd medical devices classification in accordance with the requirements of the medical device. This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) addresses the. “‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit,. Its purpose is. Principles Of In Vitro Diagnostic Medical Devices Classification.
From www.researchgate.net
Parameters for classification for In vitro diagnostic medical devices Principles Of In Vitro Diagnostic Medical Devices Classification This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) addresses the. Principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) The new ivdr aligns the regulatory approach for ivd medical devices with that of the medical devices in general by establishing a set of risk. This document describes the. Principles Of In Vitro Diagnostic Medical Devices Classification.
From mdrc-consulting.com
Downloads MDRC Principles Of In Vitro Diagnostic Medical Devices Classification Its purpose is to assist a. This guidance document is one of a series that together describe a global regulatory model for medical devices. “‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit,. The new ivdr aligns the regulatory approach for ivd medical devices with that of the medical devices. Principles Of In Vitro Diagnostic Medical Devices Classification.
From www.scribd.com
Guideline For Classification of in Vitro Diagnostic Medical Devices Principles Of In Vitro Diagnostic Medical Devices Classification The new ivdr aligns the regulatory approach for ivd medical devices with that of the medical devices in general by establishing a set of risk. Principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) Its purpose is to assist a. This guidance document is one of a series that together describe a global regulatory model for medical. Principles Of In Vitro Diagnostic Medical Devices Classification.
From formiventos.com
MDCG 202016 rev.2 Guidance on Classification Rules for in vitro Principles Of In Vitro Diagnostic Medical Devices Classification This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) addresses the. Principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) This guidance document is one of a series that together describe a global regulatory model for medical devices. Its purpose is to assist a. “‘in vitro diagnostic medical device’. Principles Of In Vitro Diagnostic Medical Devices Classification.
From www.youtube.com
Understanding the IN VITRO DIAGNOSTIC REGULATION (IVDR) Everything You Principles Of In Vitro Diagnostic Medical Devices Classification Its purpose is to assist a. Principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) This guidance document is one of a series that together describe a global regulatory model for medical devices. “‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit,. The new ivdr aligns the. Principles Of In Vitro Diagnostic Medical Devices Classification.
From www.scribd.com
Imdrf Principles of in Vitro Diagnostic (IVD) Medical Devices Principles Of In Vitro Diagnostic Medical Devices Classification The new ivdr aligns the regulatory approach for ivd medical devices with that of the medical devices in general by establishing a set of risk. Its purpose is to assist a. “‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit,. This guidance document is one of a series that together. Principles Of In Vitro Diagnostic Medical Devices Classification.
From vyomusconsulting.com
MedicalDevice & IVD Registration Process Overview Principles Of In Vitro Diagnostic Medical Devices Classification This guidance document is one of a series that together describe a global regulatory model for medical devices. Principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) “‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit,. This document describes the principles of ivd medical devices classification in. Principles Of In Vitro Diagnostic Medical Devices Classification.
From qbdgroup.com
IVDR classification of invitro diagnostic medical devices a brief guide Principles Of In Vitro Diagnostic Medical Devices Classification Its purpose is to assist a. Principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) This document describes the principles of ivd medical devices classification in accordance with the requirements of the medical device. The new ivdr aligns the regulatory approach for ivd medical devices with that of the medical devices in general by establishing a set. Principles Of In Vitro Diagnostic Medical Devices Classification.
From www.mdpi.com
Processes Free FullText Methods and Advances in the Design Principles Of In Vitro Diagnostic Medical Devices Classification “‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit,. This document describes the principles of ivd medical devices classification in accordance with the requirements of the medical device. This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) addresses the. The new ivdr. Principles Of In Vitro Diagnostic Medical Devices Classification.
From www.tuvsud.cn
Infographic The In Vitro Diagnostic Medical Device Regulation TÜV南德 Principles Of In Vitro Diagnostic Medical Devices Classification Principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) This document describes the principles of ivd medical devices classification in accordance with the requirements of the medical device. This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) addresses the. The new ivdr aligns the regulatory approach for ivd medical. Principles Of In Vitro Diagnostic Medical Devices Classification.