Purification Process Development . In the past few decades, monoclonal antibodies have been approved for therapeutic use and verified to be efficacious in. Downstream process development and manufacturing play a crucial role to ensure the protein drug’s safety, quality, identity, purity, and efficacy (sqipe) meet. This paper describes a methodology for developing chromatographic purification steps to remove very high. This review provides an overview of downstream process development strategies and tools used within the (bio)pharmaceutical. In this review of current methodology used in recovery and purification process development for mabs, basic unit. Here, we describe novel approaches for purification process development that incorporate biothermodynamics, modern high throughput. In this article, different effective strategies in biopharmaceutical purification process development are reviewed that can analogously be used for the new generation of.
from www.chromacon.com
Downstream process development and manufacturing play a crucial role to ensure the protein drug’s safety, quality, identity, purity, and efficacy (sqipe) meet. This review provides an overview of downstream process development strategies and tools used within the (bio)pharmaceutical. In this review of current methodology used in recovery and purification process development for mabs, basic unit. Here, we describe novel approaches for purification process development that incorporate biothermodynamics, modern high throughput. This paper describes a methodology for developing chromatographic purification steps to remove very high. In the past few decades, monoclonal antibodies have been approved for therapeutic use and verified to be efficacious in. In this article, different effective strategies in biopharmaceutical purification process development are reviewed that can analogously be used for the new generation of.
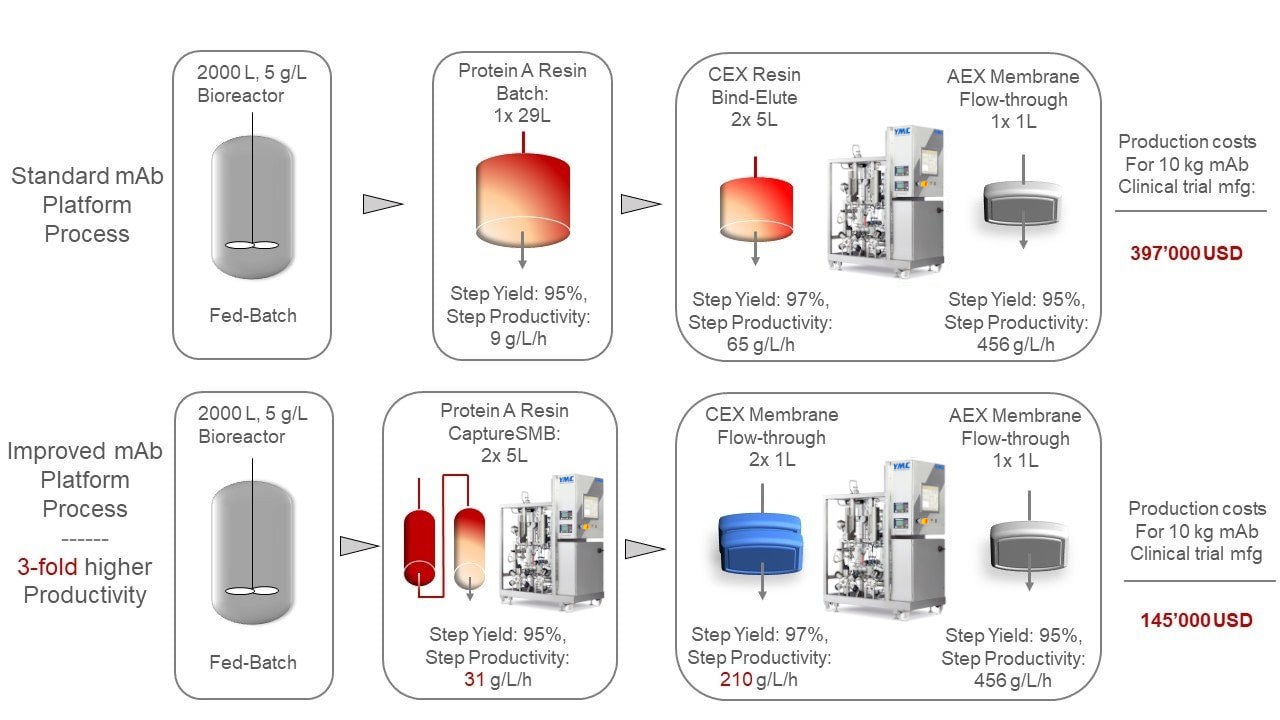
CaptureSMB for Continuous mAb Purification
Purification Process Development Downstream process development and manufacturing play a crucial role to ensure the protein drug’s safety, quality, identity, purity, and efficacy (sqipe) meet. Here, we describe novel approaches for purification process development that incorporate biothermodynamics, modern high throughput. This review provides an overview of downstream process development strategies and tools used within the (bio)pharmaceutical. Downstream process development and manufacturing play a crucial role to ensure the protein drug’s safety, quality, identity, purity, and efficacy (sqipe) meet. This paper describes a methodology for developing chromatographic purification steps to remove very high. In this review of current methodology used in recovery and purification process development for mabs, basic unit. In this article, different effective strategies in biopharmaceutical purification process development are reviewed that can analogously be used for the new generation of. In the past few decades, monoclonal antibodies have been approved for therapeutic use and verified to be efficacious in.
From stock.adobe.com
Water purification system with labeled filtration stages outline Purification Process Development This review provides an overview of downstream process development strategies and tools used within the (bio)pharmaceutical. In this review of current methodology used in recovery and purification process development for mabs, basic unit. This paper describes a methodology for developing chromatographic purification steps to remove very high. In the past few decades, monoclonal antibodies have been approved for therapeutic use. Purification Process Development.
From vectormine.com
Water purification plant filtration process explanation vector Purification Process Development Here, we describe novel approaches for purification process development that incorporate biothermodynamics, modern high throughput. This paper describes a methodology for developing chromatographic purification steps to remove very high. Downstream process development and manufacturing play a crucial role to ensure the protein drug’s safety, quality, identity, purity, and efficacy (sqipe) meet. In this article, different effective strategies in biopharmaceutical purification. Purification Process Development.
From europepmc.org
Recovery and purification process development for monoclonal antibody Purification Process Development This paper describes a methodology for developing chromatographic purification steps to remove very high. Downstream process development and manufacturing play a crucial role to ensure the protein drug’s safety, quality, identity, purity, and efficacy (sqipe) meet. This review provides an overview of downstream process development strategies and tools used within the (bio)pharmaceutical. In the past few decades, monoclonal antibodies have. Purification Process Development.
From www.researchgate.net
Biopharmaceuticals recovery and purification steps (downstream Purification Process Development This review provides an overview of downstream process development strategies and tools used within the (bio)pharmaceutical. In the past few decades, monoclonal antibodies have been approved for therapeutic use and verified to be efficacious in. In this review of current methodology used in recovery and purification process development for mabs, basic unit. This paper describes a methodology for developing chromatographic. Purification Process Development.
From www.researchgate.net
Schematic of the water purification process by drinking water company Purification Process Development In the past few decades, monoclonal antibodies have been approved for therapeutic use and verified to be efficacious in. This review provides an overview of downstream process development strategies and tools used within the (bio)pharmaceutical. In this article, different effective strategies in biopharmaceutical purification process development are reviewed that can analogously be used for the new generation of. Downstream process. Purification Process Development.
From www.researchgate.net
Downstream processing sequences for the recovery, purification, and Purification Process Development This review provides an overview of downstream process development strategies and tools used within the (bio)pharmaceutical. In the past few decades, monoclonal antibodies have been approved for therapeutic use and verified to be efficacious in. In this article, different effective strategies in biopharmaceutical purification process development are reviewed that can analogously be used for the new generation of. In this. Purification Process Development.
From www.3mireland.ie
Protein Manufacturing Biopharmaceutical Purification 3M Purification Process Development In this article, different effective strategies in biopharmaceutical purification process development are reviewed that can analogously be used for the new generation of. Here, we describe novel approaches for purification process development that incorporate biothermodynamics, modern high throughput. In the past few decades, monoclonal antibodies have been approved for therapeutic use and verified to be efficacious in. This review provides. Purification Process Development.
From www.takisbiotech.it
HIGH THROUGHPUT ANTIBODY PRODUCTION Purification Process Development In the past few decades, monoclonal antibodies have been approved for therapeutic use and verified to be efficacious in. This review provides an overview of downstream process development strategies and tools used within the (bio)pharmaceutical. Downstream process development and manufacturing play a crucial role to ensure the protein drug’s safety, quality, identity, purity, and efficacy (sqipe) meet. In this review. Purification Process Development.
From blog.interchim.com
Protein Purification, all you need to know about CEP Strategy Purification Process Development Here, we describe novel approaches for purification process development that incorporate biothermodynamics, modern high throughput. In this article, different effective strategies in biopharmaceutical purification process development are reviewed that can analogously be used for the new generation of. In this review of current methodology used in recovery and purification process development for mabs, basic unit. This review provides an overview. Purification Process Development.
From teslahealthylife.com
Stages of Purification Process Purification Process Development In this article, different effective strategies in biopharmaceutical purification process development are reviewed that can analogously be used for the new generation of. In this review of current methodology used in recovery and purification process development for mabs, basic unit. In the past few decades, monoclonal antibodies have been approved for therapeutic use and verified to be efficacious in. Here,. Purification Process Development.
From www.chromacon.com
CaptureSMB for Continuous mAb Purification Purification Process Development In this review of current methodology used in recovery and purification process development for mabs, basic unit. In the past few decades, monoclonal antibodies have been approved for therapeutic use and verified to be efficacious in. Downstream process development and manufacturing play a crucial role to ensure the protein drug’s safety, quality, identity, purity, and efficacy (sqipe) meet. Here, we. Purification Process Development.
From www.researchgate.net
Flow diagram of the purification process. Download Scientific Diagram Purification Process Development In this article, different effective strategies in biopharmaceutical purification process development are reviewed that can analogously be used for the new generation of. Downstream process development and manufacturing play a crucial role to ensure the protein drug’s safety, quality, identity, purity, and efficacy (sqipe) meet. Here, we describe novel approaches for purification process development that incorporate biothermodynamics, modern high throughput.. Purification Process Development.
From kapsaqua.com
Kaps Aqua Purification Process Purification Process Development Here, we describe novel approaches for purification process development that incorporate biothermodynamics, modern high throughput. In this review of current methodology used in recovery and purification process development for mabs, basic unit. This paper describes a methodology for developing chromatographic purification steps to remove very high. In the past few decades, monoclonal antibodies have been approved for therapeutic use and. Purification Process Development.
From www.slideserve.com
PPT A new, integrated, continuous purification process template for Purification Process Development This paper describes a methodology for developing chromatographic purification steps to remove very high. In this article, different effective strategies in biopharmaceutical purification process development are reviewed that can analogously be used for the new generation of. This review provides an overview of downstream process development strategies and tools used within the (bio)pharmaceutical. Downstream process development and manufacturing play a. Purification Process Development.
From medium.com
Different Stages of Filtration Process that an RO Water Purifier Purification Process Development This paper describes a methodology for developing chromatographic purification steps to remove very high. This review provides an overview of downstream process development strategies and tools used within the (bio)pharmaceutical. In the past few decades, monoclonal antibodies have been approved for therapeutic use and verified to be efficacious in. In this article, different effective strategies in biopharmaceutical purification process development. Purification Process Development.
From www.masterflex.com
Purification Process Workflow Products from Masterflex Purification Process Development In the past few decades, monoclonal antibodies have been approved for therapeutic use and verified to be efficacious in. In this review of current methodology used in recovery and purification process development for mabs, basic unit. In this article, different effective strategies in biopharmaceutical purification process development are reviewed that can analogously be used for the new generation of. Here,. Purification Process Development.
From www.researchgate.net
Schematic diagram of the BPE purification process. Download Purification Process Development Downstream process development and manufacturing play a crucial role to ensure the protein drug’s safety, quality, identity, purity, and efficacy (sqipe) meet. This paper describes a methodology for developing chromatographic purification steps to remove very high. In this article, different effective strategies in biopharmaceutical purification process development are reviewed that can analogously be used for the new generation of. Here,. Purification Process Development.
From onlyscientific.store
Acid Purification Only Scientific Purification Process Development This review provides an overview of downstream process development strategies and tools used within the (bio)pharmaceutical. This paper describes a methodology for developing chromatographic purification steps to remove very high. In this review of current methodology used in recovery and purification process development for mabs, basic unit. Downstream process development and manufacturing play a crucial role to ensure the protein. Purification Process Development.
From www.researchgate.net
Schematic diagram of the purification process (a) Process flow for Purification Process Development In this review of current methodology used in recovery and purification process development for mabs, basic unit. This paper describes a methodology for developing chromatographic purification steps to remove very high. In this article, different effective strategies in biopharmaceutical purification process development are reviewed that can analogously be used for the new generation of. In the past few decades, monoclonal. Purification Process Development.
From www.researchgate.net
(PDF) Accelerating purification process development of an early phase Purification Process Development This review provides an overview of downstream process development strategies and tools used within the (bio)pharmaceutical. Here, we describe novel approaches for purification process development that incorporate biothermodynamics, modern high throughput. In this review of current methodology used in recovery and purification process development for mabs, basic unit. In the past few decades, monoclonal antibodies have been approved for therapeutic. Purification Process Development.
From www.researchgate.net
Schematic diagram of the purification process (a) Process flow for Purification Process Development This review provides an overview of downstream process development strategies and tools used within the (bio)pharmaceutical. This paper describes a methodology for developing chromatographic purification steps to remove very high. In this review of current methodology used in recovery and purification process development for mabs, basic unit. In this article, different effective strategies in biopharmaceutical purification process development are reviewed. Purification Process Development.
From www.youtube.com
PURIFICATION OF WATER PURIFICATION METHODS SCIENCE EDUCATIONAL Purification Process Development Downstream process development and manufacturing play a crucial role to ensure the protein drug’s safety, quality, identity, purity, and efficacy (sqipe) meet. Here, we describe novel approaches for purification process development that incorporate biothermodynamics, modern high throughput. In this article, different effective strategies in biopharmaceutical purification process development are reviewed that can analogously be used for the new generation of.. Purification Process Development.
From softflowwater.com
Purification Process By Soft Flow Water In San Diego, CA Purification Process Development Here, we describe novel approaches for purification process development that incorporate biothermodynamics, modern high throughput. Downstream process development and manufacturing play a crucial role to ensure the protein drug’s safety, quality, identity, purity, and efficacy (sqipe) meet. In the past few decades, monoclonal antibodies have been approved for therapeutic use and verified to be efficacious in. This paper describes a. Purification Process Development.
From www.frontiersin.org
Frontiers Evaluating the Cost of Pharmaceutical Purification for a Purification Process Development Here, we describe novel approaches for purification process development that incorporate biothermodynamics, modern high throughput. In this article, different effective strategies in biopharmaceutical purification process development are reviewed that can analogously be used for the new generation of. This paper describes a methodology for developing chromatographic purification steps to remove very high. Downstream process development and manufacturing play a crucial. Purification Process Development.
From www.britannica.com
Water purification Description, Processes, & Importance Britannica Purification Process Development This review provides an overview of downstream process development strategies and tools used within the (bio)pharmaceutical. In this article, different effective strategies in biopharmaceutical purification process development are reviewed that can analogously be used for the new generation of. This paper describes a methodology for developing chromatographic purification steps to remove very high. Here, we describe novel approaches for purification. Purification Process Development.
From www.kbibiopharma.com
Development of effective purification processes for bispecifics using Purification Process Development This review provides an overview of downstream process development strategies and tools used within the (bio)pharmaceutical. In this article, different effective strategies in biopharmaceutical purification process development are reviewed that can analogously be used for the new generation of. Downstream process development and manufacturing play a crucial role to ensure the protein drug’s safety, quality, identity, purity, and efficacy (sqipe). Purification Process Development.
From teslahealthylife.com
Stages of Purification Process Purification Process Development This review provides an overview of downstream process development strategies and tools used within the (bio)pharmaceutical. Downstream process development and manufacturing play a crucial role to ensure the protein drug’s safety, quality, identity, purity, and efficacy (sqipe) meet. In the past few decades, monoclonal antibodies have been approved for therapeutic use and verified to be efficacious in. In this article,. Purification Process Development.
From www.researchgate.net
Schematic of platform mAb purification process. Download Scientific Purification Process Development This paper describes a methodology for developing chromatographic purification steps to remove very high. This review provides an overview of downstream process development strategies and tools used within the (bio)pharmaceutical. In this article, different effective strategies in biopharmaceutical purification process development are reviewed that can analogously be used for the new generation of. Downstream process development and manufacturing play a. Purification Process Development.
From www.kth.se
beadbased purification KTH Purification Process Development This review provides an overview of downstream process development strategies and tools used within the (bio)pharmaceutical. In the past few decades, monoclonal antibodies have been approved for therapeutic use and verified to be efficacious in. In this article, different effective strategies in biopharmaceutical purification process development are reviewed that can analogously be used for the new generation of. This paper. Purification Process Development.
From www.scorpiusbiologics.com
Downstream Purification Process Development USA Process Development Purification Process Development Downstream process development and manufacturing play a crucial role to ensure the protein drug’s safety, quality, identity, purity, and efficacy (sqipe) meet. This review provides an overview of downstream process development strategies and tools used within the (bio)pharmaceutical. Here, we describe novel approaches for purification process development that incorporate biothermodynamics, modern high throughput. This paper describes a methodology for developing. Purification Process Development.
From www.vrogue.co
7 Stages Of Water Purification Process 2021 vrogue.co Purification Process Development In this review of current methodology used in recovery and purification process development for mabs, basic unit. In this article, different effective strategies in biopharmaceutical purification process development are reviewed that can analogously be used for the new generation of. This review provides an overview of downstream process development strategies and tools used within the (bio)pharmaceutical. This paper describes a. Purification Process Development.
From www.synthace.com
Purification Process Development Synthace Purification Process Development Here, we describe novel approaches for purification process development that incorporate biothermodynamics, modern high throughput. This review provides an overview of downstream process development strategies and tools used within the (bio)pharmaceutical. This paper describes a methodology for developing chromatographic purification steps to remove very high. In this article, different effective strategies in biopharmaceutical purification process development are reviewed that can. Purification Process Development.
From www.cytivalifesciences.com
Gene therapy AAV vector purification process Cytiva Purification Process Development This paper describes a methodology for developing chromatographic purification steps to remove very high. In the past few decades, monoclonal antibodies have been approved for therapeutic use and verified to be efficacious in. In this article, different effective strategies in biopharmaceutical purification process development are reviewed that can analogously be used for the new generation of. In this review of. Purification Process Development.
From www.mdpi.com
JCM Free FullText Development of a SingleStep AntibodyDrug Purification Process Development This paper describes a methodology for developing chromatographic purification steps to remove very high. This review provides an overview of downstream process development strategies and tools used within the (bio)pharmaceutical. Downstream process development and manufacturing play a crucial role to ensure the protein drug’s safety, quality, identity, purity, and efficacy (sqipe) meet. In the past few decades, monoclonal antibodies have. Purification Process Development.
From www.genengnews.com
PhaseDependent Approaches to Plasmid DNA Manufacture Purification Process Development In this review of current methodology used in recovery and purification process development for mabs, basic unit. This review provides an overview of downstream process development strategies and tools used within the (bio)pharmaceutical. Downstream process development and manufacturing play a crucial role to ensure the protein drug’s safety, quality, identity, purity, and efficacy (sqipe) meet. This paper describes a methodology. Purification Process Development.