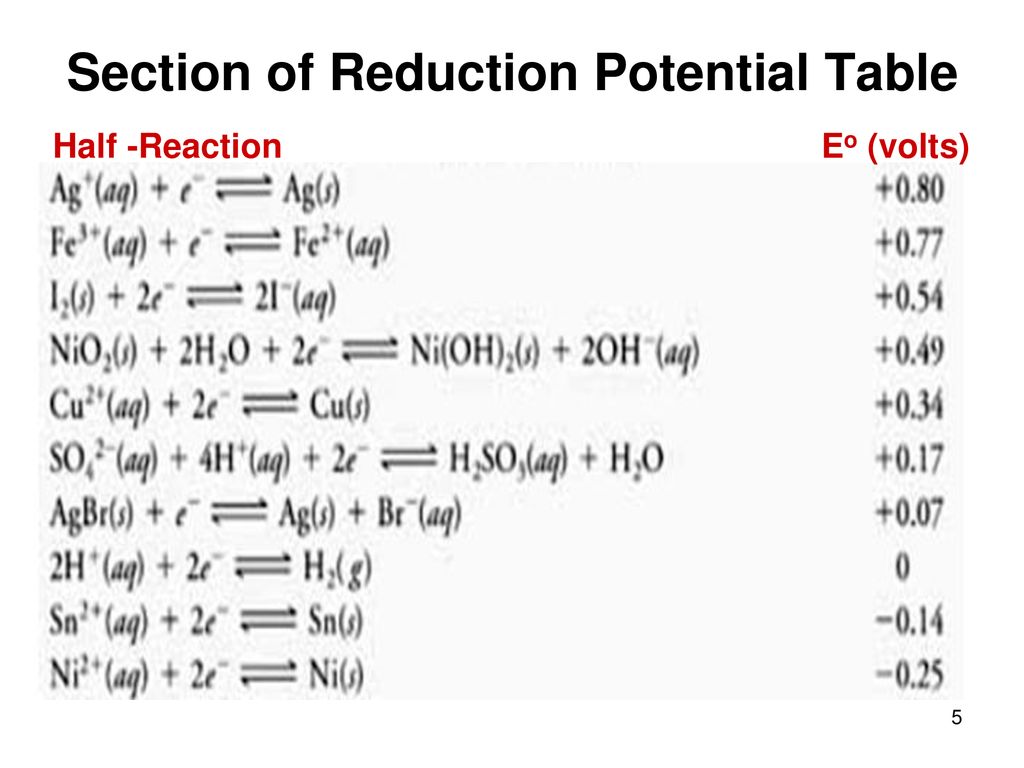
Standard Reduction Potential Units . Use standard reduction potentials to determine the. Describe and relate the definitions of electrode and cell potentials. Periodic table of the elements. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. Selected acid dissociation constants at 25°c. Use standard reduction potentials to determine the better. By the end of this section, you will be able to: Interpret electrode potentials in terms of relative oxidant and reductant. Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s) + 4oh −.
from slideplayer.com
Selected acid dissociation constants at 25°c. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. Interpret electrode potentials in terms of relative oxidant and reductant. Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s) + 4oh −. Periodic table of the elements. Use standard reduction potentials to determine the better. By the end of this section, you will be able to: Use standard reduction potentials to determine the. Describe and relate the definitions of electrode and cell potentials.
Electrochemistry Part III Reduction Potentials ppt download
Standard Reduction Potential Units The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s) + 4oh −. Use standard reduction potentials to determine the better. Interpret electrode potentials in terms of relative oxidant and reductant. Use standard reduction potentials to determine the. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. Describe and relate the definitions of electrode and cell potentials. Periodic table of the elements. Selected acid dissociation constants at 25°c. By the end of this section, you will be able to:
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Units Periodic table of the elements. Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s) + 4oh −. Use standard reduction potentials to determine the better. By the end of this section, you will be able to: Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. Selected acid dissociation. Standard Reduction Potential Units.
From slideplayer.com
Electrochemistry Part III Reduction Potentials ppt download Standard Reduction Potential Units Periodic table of the elements. Describe and relate the definitions of electrode and cell potentials. By the end of this section, you will be able to: Use standard reduction potentials to determine the. Interpret electrode potentials in terms of relative oxidant and reductant. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured. Standard Reduction Potential Units.
From www.bartleby.com
Answered Selected Standard Reduction Potentials… bartleby Standard Reduction Potential Units Periodic table of the elements. Selected acid dissociation constants at 25°c. Use standard reduction potentials to determine the better. Describe and relate the definitions of electrode and cell potentials. Use standard reduction potentials to determine the. By the end of this section, you will be able to: Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s) + 4oh −.. Standard Reduction Potential Units.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Units The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. Use standard reduction potentials to determine the better. Periodic table of the elements. By the end of this section,. Standard Reduction Potential Units.
From www.scribd.com
Standard Reduction Potentials Data Extended pdf Standard Reduction Potential Units Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. By the end of this section, you will be able to: Periodic table of the elements. Al (oh) 4−. Standard Reduction Potential Units.
From www.pveducation.org
Standard Potential PVEducation Standard Reduction Potential Units Periodic table of the elements. Selected acid dissociation constants at 25°c. Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s) + 4oh −. Interpret electrode potentials in terms of relative oxidant and reductant. By the end of this section, you will be able to: The standard reduction potential is the tendency for a chemical species to be reduced, and. Standard Reduction Potential Units.
From www.slideserve.com
PPT Intersection 13 PowerPoint Presentation, free download ID567059 Standard Reduction Potential Units Interpret electrode potentials in terms of relative oxidant and reductant. Use standard reduction potentials to determine the better. By the end of this section, you will be able to: Periodic table of the elements. Describe and relate the definitions of electrode and cell potentials. Use standard reduction potentials to determine the. Selected acid dissociation constants at 25°c. The standard reduction. Standard Reduction Potential Units.
From testbook.com
Reduction Potential Measurement, Half Cells, Differences & More Standard Reduction Potential Units Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. Use standard reduction potentials to determine the better. By the end of this section, you will be able to:. Standard Reduction Potential Units.
From www.researchgate.net
A) Standard redox potentials (E 0 ) for half reactions that can occur Standard Reduction Potential Units Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s) + 4oh −. Periodic table of the elements. Interpret electrode potentials in terms of relative oxidant and reductant. Selected acid dissociation constants at 25°c. Use standard reduction potentials to determine the better. Use standard reduction potentials to determine the. The standard reduction potential is the tendency for a chemical species. Standard Reduction Potential Units.
From www.slideserve.com
PPT 21 Electrochemistry PowerPoint Presentation, free download ID Standard Reduction Potential Units Selected acid dissociation constants at 25°c. Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s) + 4oh −. Periodic table of the elements. Interpret electrode potentials in terms of relative oxidant and reductant. Use standard reduction potentials to determine the. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts. Standard Reduction Potential Units.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID6601383 Standard Reduction Potential Units The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. Describe and relate the definitions of electrode and cell potentials. Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s) + 4oh −. Selected acid dissociation constants at 25°c. Use standard reduction potentials to determine the. Interpret electrode potentials. Standard Reduction Potential Units.
From www.slideserve.com
PPT Oxidation and Reduction PowerPoint Presentation, free download Standard Reduction Potential Units Use standard reduction potentials to determine the. By the end of this section, you will be able to: Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. Selected acid dissociation constants at 25°c. Use standard reduction potentials to determine the better. Al (oh) 4− + 3 e− \ (\rightleftharpoons\). Standard Reduction Potential Units.
From pressbooks.uiowa.edu
Topic 11 Appendix A Standard Reduction Potentials at 25ºC CHEM 1120 Standard Reduction Potential Units Use standard reduction potentials to determine the better. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. Interpret electrode potentials in terms of relative oxidant and reductant. By the end of this section, you will be able to: Describe and relate the definitions of electrode and cell potentials.. Standard Reduction Potential Units.
From www.slideserve.com
PPT Redox Reactions and Electrochemistry PowerPoint Presentation Standard Reduction Potential Units Describe and relate the definitions of electrode and cell potentials. Use standard reduction potentials to determine the. Use standard reduction potentials to determine the better. Periodic table of the elements. Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s) + 4oh −. Interpret electrode potentials in terms of relative oxidant and reductant. Selected acid dissociation constants at 25°c. The. Standard Reduction Potential Units.
From www.youtube.com
Standard reduction potentials Redox reactions and electrochemistry Standard Reduction Potential Units The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. Use standard reduction potentials to determine the better. Interpret electrode potentials in terms of relative oxidant and reductant. Use standard reduction potentials to determine the. Describe and relate the definitions of electrode and cell potentials. Al (oh) 4− +. Standard Reduction Potential Units.
From www.pinterest.com
Standard Reduction Potentials Reduction potential, Equations Standard Reduction Potential Units The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. Describe and relate the definitions of electrode and cell potentials. Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s) + 4oh −. Selected acid dissociation constants at 25°c. Periodic table of the elements. By the end of this. Standard Reduction Potential Units.
From www.slideserve.com
PPT Product stabilizations in hydrolysis PowerPoint Presentation Standard Reduction Potential Units Use standard reduction potentials to determine the better. By the end of this section, you will be able to: Interpret electrode potentials in terms of relative oxidant and reductant. Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s) + 4oh −. Periodic table of the elements. Selected acid dissociation constants at 25°c. Describe and relate the definitions of electrode. Standard Reduction Potential Units.
From mavink.com
Standard Reduction Potentials Standard Reduction Potential Units Periodic table of the elements. Use standard reduction potentials to determine the. Describe and relate the definitions of electrode and cell potentials. By the end of this section, you will be able to: Selected acid dissociation constants at 25°c. Use standard reduction potentials to determine the better. Interpret electrode potentials in terms of relative oxidant and reductant. The standard reduction. Standard Reduction Potential Units.
From www.slideserve.com
PPT Oxidation and Reduction PowerPoint Presentation, free download Standard Reduction Potential Units Periodic table of the elements. By the end of this section, you will be able to: Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s) + 4oh −. Selected acid dissociation constants at 25°c. The standard reduction potential is. Standard Reduction Potential Units.
From www.slideserve.com
PPT Electrochemistry Part III Reduction Potentials PowerPoint Standard Reduction Potential Units Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s) + 4oh −. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. Describe and relate the definitions of electrode and cell potentials. Use standard reduction potentials to determine the. Periodic table of the elements. Use standard reduction potentials. Standard Reduction Potential Units.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Units Selected acid dissociation constants at 25°c. Use standard reduction potentials to determine the better. Use standard reduction potentials to determine the. By the end of this section, you will be able to: Interpret electrode potentials in terms of relative oxidant and reductant. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in. Standard Reduction Potential Units.
From ch302.cm.utexas.edu
Standard Reduction Potentials Standard Reduction Potential Units Use standard reduction potentials to determine the better. Periodic table of the elements. Selected acid dissociation constants at 25°c. Describe and relate the definitions of electrode and cell potentials. Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s) + 4oh −. Interpret electrode potentials in terms of relative oxidant and reductant. By the end of this section, you will. Standard Reduction Potential Units.
From ar.inspiredpencil.com
Standard Reduction Potential Table Standard Reduction Potential Units The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. By the end of this section, you will be able to: Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s) + 4oh −. Use standard reduction potentials to determine the. Periodic table of the elements. Interpret electrode potentials. Standard Reduction Potential Units.
From www.slideserve.com
PPT ELECTROCHEMISTRY Chapter 21 PowerPoint Presentation, free Standard Reduction Potential Units Interpret electrode potentials in terms of relative oxidant and reductant. Use standard reduction potentials to determine the. Describe and relate the definitions of electrode and cell potentials. Use standard reduction potentials to determine the better. Periodic table of the elements. Selected acid dissociation constants at 25°c. The standard reduction potential is the tendency for a chemical species to be reduced,. Standard Reduction Potential Units.
From www.researchgate.net
Gibbs free energy changes and standard reduction potentials at 25 • C Standard Reduction Potential Units Selected acid dissociation constants at 25°c. Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s) + 4oh −. Periodic table of the elements. Use standard reduction potentials to determine the. By the end of this section, you will be able to: The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in. Standard Reduction Potential Units.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Units Interpret electrode potentials in terms of relative oxidant and reductant. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. Periodic table of the elements. Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s) + 4oh −. Describe and relate the definitions of electrode and cell potentials. By. Standard Reduction Potential Units.
From www.youtube.com
Standard Reduction Potential YouTube Standard Reduction Potential Units The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. Use standard reduction potentials to determine the better. Use standard reduction potentials to determine the. Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s) + 4oh −. Interpret electrode potentials in terms of relative oxidant and reductant. By. Standard Reduction Potential Units.
From ecampusontario.pressbooks.pub
Appendix 4 Standard Reduction Potentials First Year General Chemistry Standard Reduction Potential Units Interpret electrode potentials in terms of relative oxidant and reductant. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. Selected acid dissociation constants at 25°c. Periodic table of the elements. Use standard reduction potentials to determine the better. Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s). Standard Reduction Potential Units.
From vpia.org.vn
Standard Reduction Potentials made easy, reduction line Standard Reduction Potential Units The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. Use standard reduction potentials to determine the. Describe and relate the definitions of electrode and cell potentials. Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s) + 4oh −. Interpret electrode potentials in terms of relative oxidant and. Standard Reduction Potential Units.
From www.flinnsci.com
Standard Reduction Potential Charts for Chemistry Standard Reduction Potential Units By the end of this section, you will be able to: Use standard reduction potentials to determine the better. Interpret electrode potentials in terms of relative oxidant and reductant. Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s) + 4oh −. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in. Standard Reduction Potential Units.
From www.solutioninn.com
[Solved] Use the table of standard reduction poten SolutionInn Standard Reduction Potential Units Describe and relate the definitions of electrode and cell potentials. By the end of this section, you will be able to: The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s) + 4oh −. Periodic table of the elements.. Standard Reduction Potential Units.
From oneclass.com
OneClass Standard reduction potentials Use the table of standard Standard Reduction Potential Units By the end of this section, you will be able to: Selected acid dissociation constants at 25°c. Describe and relate the definitions of electrode and cell potentials. Use standard reduction potentials to determine the. Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s) + 4oh −. Interpret electrode potentials in terms of relative oxidant and reductant. The standard reduction. Standard Reduction Potential Units.
From ch302.cm.utexas.edu
Electrochemistry_Reduction_Potentials Standard Reduction Potential Units By the end of this section, you will be able to: Use standard reduction potentials to determine the better. Describe and relate the definitions of electrode and cell potentials. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s). Standard Reduction Potential Units.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Units Describe and relate the definitions of electrode and cell potentials. Al (oh) 4− + 3 e− \ (\rightleftharpoons\) al (s) + 4oh −. Selected acid dissociation constants at 25°c. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. By the end of this section, you will be able. Standard Reduction Potential Units.
From www.bartleby.com
Answered Standard reduction potentials for… bartleby Standard Reduction Potential Units Describe and relate the definitions of electrode and cell potentials. Selected acid dissociation constants at 25°c. Use standard reduction potentials to determine the. Periodic table of the elements. By the end of this section, you will be able to: Use standard reduction potentials to determine the better. The standard reduction potential is the tendency for a chemical species to be. Standard Reduction Potential Units.