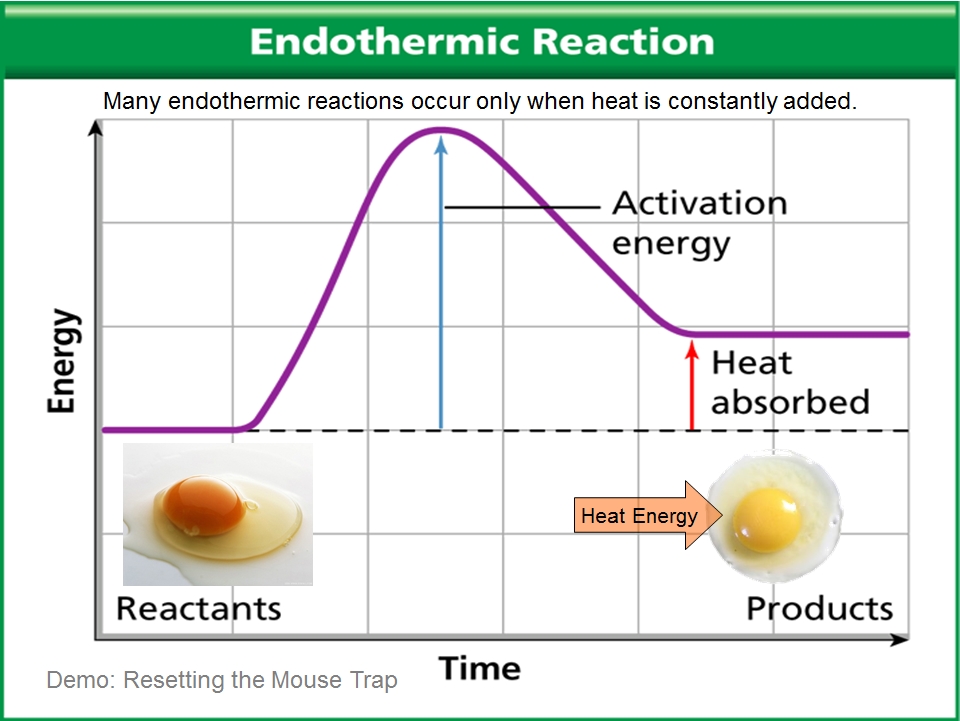
Is Water Boiling An Exothermic Process . therefore, boiling water is an endothermic process. If the system gains a certain amount of energy, that energy is supplied by the surroundings. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. The applied heat helps break the intermolecular bonds between the water molecules, which spreads in the molecules further apart. the reaction converting wood to carbon dioxide and water (among other things) continues, releasing heat energy in. The water turns completely to steam (water vapor) when all the intermolecular bonds are broken. no, the boiling of water is not exothermic; an exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy change. This means it absorbs heat from its surroundings. This is because energy is. This means it absorbs heat from its surroundings. boiling water is an endothermic process. if the system loses a certain amount of energy, that same amount of energy is gained by the surroundings. because we must add heat, boiling water is a process that chemists call endothermic.
from vhmsscience.weebly.com
This means it absorbs heat from its surroundings. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. if the system loses a certain amount of energy, that same amount of energy is gained by the surroundings. The water turns completely to steam (water vapor) when all the intermolecular bonds are broken. boiling water is an endothermic process. because we must add heat, boiling water is a process that chemists call endothermic. The applied heat helps break the intermolecular bonds between the water molecules, which spreads in the molecules further apart. If the system gains a certain amount of energy, that energy is supplied by the surroundings. therefore, boiling water is an endothermic process. no, the boiling of water is not exothermic;
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE
Is Water Boiling An Exothermic Process because we must add heat, boiling water is a process that chemists call endothermic. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. boiling water is an endothermic process. no, the boiling of water is not exothermic; This means it absorbs heat from its surroundings. This is because energy is. The water turns completely to steam (water vapor) when all the intermolecular bonds are broken. The applied heat helps break the intermolecular bonds between the water molecules, which spreads in the molecules further apart. the reaction converting wood to carbon dioxide and water (among other things) continues, releasing heat energy in. an exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy change. because we must add heat, boiling water is a process that chemists call endothermic. If the system gains a certain amount of energy, that energy is supplied by the surroundings. This means it absorbs heat from its surroundings. therefore, boiling water is an endothermic process. if the system loses a certain amount of energy, that same amount of energy is gained by the surroundings.
From saliqsareeta.blogspot.com
26+ Exothermic Reaction Energy Diagram SaliqSareeta Is Water Boiling An Exothermic Process This means it absorbs heat from its surroundings. This is because energy is. an exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy change. if the system loses a certain amount of energy, that same amount of energy is gained by the surroundings. This means it absorbs heat from its. Is Water Boiling An Exothermic Process.
From sciencenotes.org
Endothermic Reactions Definition and Examples Is Water Boiling An Exothermic Process The water turns completely to steam (water vapor) when all the intermolecular bonds are broken. boiling water is an endothermic process. no, the boiling of water is not exothermic; If the system gains a certain amount of energy, that energy is supplied by the surroundings. A chemical reaction or physical change is endothermic if heat is absorbed by. Is Water Boiling An Exothermic Process.
From slideplayer.com
Chapter 11 Liquids, solids, and intermolecular forces ppt download Is Water Boiling An Exothermic Process if the system loses a certain amount of energy, that same amount of energy is gained by the surroundings. no, the boiling of water is not exothermic; This is because energy is. boiling water is an endothermic process. an exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy. Is Water Boiling An Exothermic Process.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Is Water Boiling An Exothermic Process therefore, boiling water is an endothermic process. The water turns completely to steam (water vapor) when all the intermolecular bonds are broken. The applied heat helps break the intermolecular bonds between the water molecules, which spreads in the molecules further apart. if the system loses a certain amount of energy, that same amount of energy is gained by. Is Water Boiling An Exothermic Process.
From examples.yourdictionary.com
Exothermic Reaction Examples Found in Real Life YourDictionary Is Water Boiling An Exothermic Process because we must add heat, boiling water is a process that chemists call endothermic. an exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy change. therefore, boiling water is an endothermic process. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the. Is Water Boiling An Exothermic Process.
From www.teachoo.com
What is the Difference between Evaporation and Boiling? Class 9 Is Water Boiling An Exothermic Process If the system gains a certain amount of energy, that energy is supplied by the surroundings. therefore, boiling water is an endothermic process. no, the boiling of water is not exothermic; This means it absorbs heat from its surroundings. an exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy. Is Water Boiling An Exothermic Process.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Is Water Boiling An Exothermic Process therefore, boiling water is an endothermic process. if the system loses a certain amount of energy, that same amount of energy is gained by the surroundings. This is because energy is. the reaction converting wood to carbon dioxide and water (among other things) continues, releasing heat energy in. The water turns completely to steam (water vapor) when. Is Water Boiling An Exothermic Process.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Temperature Change Is Water Boiling An Exothermic Process This is because energy is. no, the boiling of water is not exothermic; The applied heat helps break the intermolecular bonds between the water molecules, which spreads in the molecules further apart. This means it absorbs heat from its surroundings. an exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy. Is Water Boiling An Exothermic Process.
From sciencenotes.org
Bond Energy and Strength Is Water Boiling An Exothermic Process The applied heat helps break the intermolecular bonds between the water molecules, which spreads in the molecules further apart. therefore, boiling water is an endothermic process. If the system gains a certain amount of energy, that energy is supplied by the surroundings. no, the boiling of water is not exothermic; A chemical reaction or physical change is endothermic. Is Water Boiling An Exothermic Process.
From kunduz.com
Exothermic Reaction Definition, Properties, Examples, Exothermic and Is Water Boiling An Exothermic Process This means it absorbs heat from its surroundings. If the system gains a certain amount of energy, that energy is supplied by the surroundings. therefore, boiling water is an endothermic process. if the system loses a certain amount of energy, that same amount of energy is gained by the surroundings. The applied heat helps break the intermolecular bonds. Is Water Boiling An Exothermic Process.
From byjus.com
State whether the following is exothermic or endothermic reaction and Is Water Boiling An Exothermic Process if the system loses a certain amount of energy, that same amount of energy is gained by the surroundings. the reaction converting wood to carbon dioxide and water (among other things) continues, releasing heat energy in. because we must add heat, boiling water is a process that chemists call endothermic. The water turns completely to steam (water. Is Water Boiling An Exothermic Process.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Is Water Boiling An Exothermic Process therefore, boiling water is an endothermic process. boiling water is an endothermic process. an exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy change. no, the boiling of water is not exothermic; This means it absorbs heat from its surroundings. because we must add heat, boiling water. Is Water Boiling An Exothermic Process.
From www.numerade.com
Boiling water is Select one a. exothermic and increasing in Is Water Boiling An Exothermic Process the reaction converting wood to carbon dioxide and water (among other things) continues, releasing heat energy in. If the system gains a certain amount of energy, that energy is supplied by the surroundings. This means it absorbs heat from its surroundings. This is because energy is. boiling water is an endothermic process. an exothermic reaction is defined. Is Water Boiling An Exothermic Process.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Is Water Boiling An Exothermic Process This means it absorbs heat from its surroundings. If the system gains a certain amount of energy, that energy is supplied by the surroundings. This means it absorbs heat from its surroundings. therefore, boiling water is an endothermic process. the reaction converting wood to carbon dioxide and water (among other things) continues, releasing heat energy in. no,. Is Water Boiling An Exothermic Process.
From sciencenotes.org
What Are the Bubbles in Boiling Water? Is Water Boiling An Exothermic Process no, the boiling of water is not exothermic; an exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy change. boiling water is an endothermic process. This means it absorbs heat from its surroundings. because we must add heat, boiling water is a process that chemists call endothermic. If. Is Water Boiling An Exothermic Process.
From en.ppt-online.org
Thermal Energy, Chemical Energy online presentation Is Water Boiling An Exothermic Process if the system loses a certain amount of energy, that same amount of energy is gained by the surroundings. The water turns completely to steam (water vapor) when all the intermolecular bonds are broken. The applied heat helps break the intermolecular bonds between the water molecules, which spreads in the molecules further apart. boiling water is an endothermic. Is Water Boiling An Exothermic Process.
From www.numerade.com
SOLVED Be sure to answer all parts Classify each process as Is Water Boiling An Exothermic Process This means it absorbs heat from its surroundings. If the system gains a certain amount of energy, that energy is supplied by the surroundings. therefore, boiling water is an endothermic process. This is because energy is. This means it absorbs heat from its surroundings. because we must add heat, boiling water is a process that chemists call endothermic.. Is Water Boiling An Exothermic Process.
From whatsinsight.org
Endothermic vs. Exothermic with Examples What's Insight Is Water Boiling An Exothermic Process no, the boiling of water is not exothermic; the reaction converting wood to carbon dioxide and water (among other things) continues, releasing heat energy in. This means it absorbs heat from its surroundings. If the system gains a certain amount of energy, that energy is supplied by the surroundings. A chemical reaction or physical change is endothermic if. Is Water Boiling An Exothermic Process.
From sciencenotes.org
Exothermic Reactions Definition and Examples Is Water Boiling An Exothermic Process The water turns completely to steam (water vapor) when all the intermolecular bonds are broken. This means it absorbs heat from its surroundings. no, the boiling of water is not exothermic; The applied heat helps break the intermolecular bonds between the water molecules, which spreads in the molecules further apart. if the system loses a certain amount of. Is Water Boiling An Exothermic Process.
From www.ck12.org
Energy Change in Reactions CK12 Foundation Is Water Boiling An Exothermic Process no, the boiling of water is not exothermic; if the system loses a certain amount of energy, that same amount of energy is gained by the surroundings. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. This means it absorbs heat from its surroundings. The applied heat helps break. Is Water Boiling An Exothermic Process.
From www.slideserve.com
PPT Endothermic vs. Exothermic Reactions PowerPoint Presentation Is Water Boiling An Exothermic Process no, the boiling of water is not exothermic; A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. the reaction converting wood to carbon dioxide and water (among other things) continues, releasing heat energy in. therefore, boiling water is an endothermic process. if the system loses a certain. Is Water Boiling An Exothermic Process.
From www.numerade.com
SOLVED Which one of the following is an exothermic process? A) ice Is Water Boiling An Exothermic Process an exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy change. if the system loses a certain amount of energy, that same amount of energy is gained by the surroundings. This means it absorbs heat from its surroundings. A chemical reaction or physical change is endothermic if heat is absorbed. Is Water Boiling An Exothermic Process.
From www.studyorgo.com
Organic Chemistry Help Tools Is Water Boiling An Exothermic Process This means it absorbs heat from its surroundings. This is because energy is. no, the boiling of water is not exothermic; The applied heat helps break the intermolecular bonds between the water molecules, which spreads in the molecules further apart. because we must add heat, boiling water is a process that chemists call endothermic. boiling water is. Is Water Boiling An Exothermic Process.
From tapwaterfeatures.com
Is Boiling Water Endothermic or Exothermic Is Water Boiling An Exothermic Process A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. boiling water is an endothermic process. no, the boiling of water is not exothermic; an exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy change. therefore, boiling water is an. Is Water Boiling An Exothermic Process.
From www.slideshare.net
Thermochemistry Is Water Boiling An Exothermic Process the reaction converting wood to carbon dioxide and water (among other things) continues, releasing heat energy in. no, the boiling of water is not exothermic; This means it absorbs heat from its surroundings. If the system gains a certain amount of energy, that energy is supplied by the surroundings. therefore, boiling water is an endothermic process. This. Is Water Boiling An Exothermic Process.
From quizizz.com
Endothermic vs Exothermic Process Chemistry Quiz Quizizz Is Water Boiling An Exothermic Process if the system loses a certain amount of energy, that same amount of energy is gained by the surroundings. no, the boiling of water is not exothermic; an exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy change. A chemical reaction or physical change is endothermic if heat is. Is Water Boiling An Exothermic Process.
From online-learning-college.com
Exothermic and endothermic reactions Key differences Is Water Boiling An Exothermic Process The applied heat helps break the intermolecular bonds between the water molecules, which spreads in the molecules further apart. an exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy change. therefore, boiling water is an endothermic process. If the system gains a certain amount of energy, that energy is supplied. Is Water Boiling An Exothermic Process.
From www.pinterest.co.uk
Endothermic and Exothermic Reactions infographic diagram showing Is Water Boiling An Exothermic Process the reaction converting wood to carbon dioxide and water (among other things) continues, releasing heat energy in. The applied heat helps break the intermolecular bonds between the water molecules, which spreads in the molecules further apart. an exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy change. A chemical reaction. Is Water Boiling An Exothermic Process.
From www.numerade.com
SOLVED Which of the following is true about phase changes? (Select all Is Water Boiling An Exothermic Process The water turns completely to steam (water vapor) when all the intermolecular bonds are broken. This means it absorbs heat from its surroundings. This is because energy is. if the system loses a certain amount of energy, that same amount of energy is gained by the surroundings. the reaction converting wood to carbon dioxide and water (among other. Is Water Boiling An Exothermic Process.
From slideplayer.com
THERMOCHEMISTRY Courtesy of ppt download Is Water Boiling An Exothermic Process the reaction converting wood to carbon dioxide and water (among other things) continues, releasing heat energy in. If the system gains a certain amount of energy, that energy is supplied by the surroundings. The applied heat helps break the intermolecular bonds between the water molecules, which spreads in the molecules further apart. therefore, boiling water is an endothermic. Is Water Boiling An Exothermic Process.
From www.chegg.com
Solved Which One Of The Following Processes Is Exothermic... Is Water Boiling An Exothermic Process because we must add heat, boiling water is a process that chemists call endothermic. The applied heat helps break the intermolecular bonds between the water molecules, which spreads in the molecules further apart. The water turns completely to steam (water vapor) when all the intermolecular bonds are broken. This is because energy is. therefore, boiling water is an. Is Water Boiling An Exothermic Process.
From exyyhvyng.blob.core.windows.net
Endothermic Reaction Bitesize at Julie Taylor blog Is Water Boiling An Exothermic Process The water turns completely to steam (water vapor) when all the intermolecular bonds are broken. boiling water is an endothermic process. This is because energy is. an exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy change. the reaction converting wood to carbon dioxide and water (among other things). Is Water Boiling An Exothermic Process.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Is Water Boiling An Exothermic Process This means it absorbs heat from its surroundings. the reaction converting wood to carbon dioxide and water (among other things) continues, releasing heat energy in. if the system loses a certain amount of energy, that same amount of energy is gained by the surroundings. If the system gains a certain amount of energy, that energy is supplied by. Is Water Boiling An Exothermic Process.
From giofvwsan.blob.core.windows.net
Why Is The Boiling Of Water A Physical Change at Ray Howard blog Is Water Boiling An Exothermic Process boiling water is an endothermic process. This is because energy is. because we must add heat, boiling water is a process that chemists call endothermic. If the system gains a certain amount of energy, that energy is supplied by the surroundings. The water turns completely to steam (water vapor) when all the intermolecular bonds are broken. no,. Is Water Boiling An Exothermic Process.
From www.slideserve.com
PPT Endothermic and exothermic reactions PowerPoint Presentation Is Water Boiling An Exothermic Process boiling water is an endothermic process. if the system loses a certain amount of energy, that same amount of energy is gained by the surroundings. If the system gains a certain amount of energy, that energy is supplied by the surroundings. therefore, boiling water is an endothermic process. because we must add heat, boiling water is. Is Water Boiling An Exothermic Process.