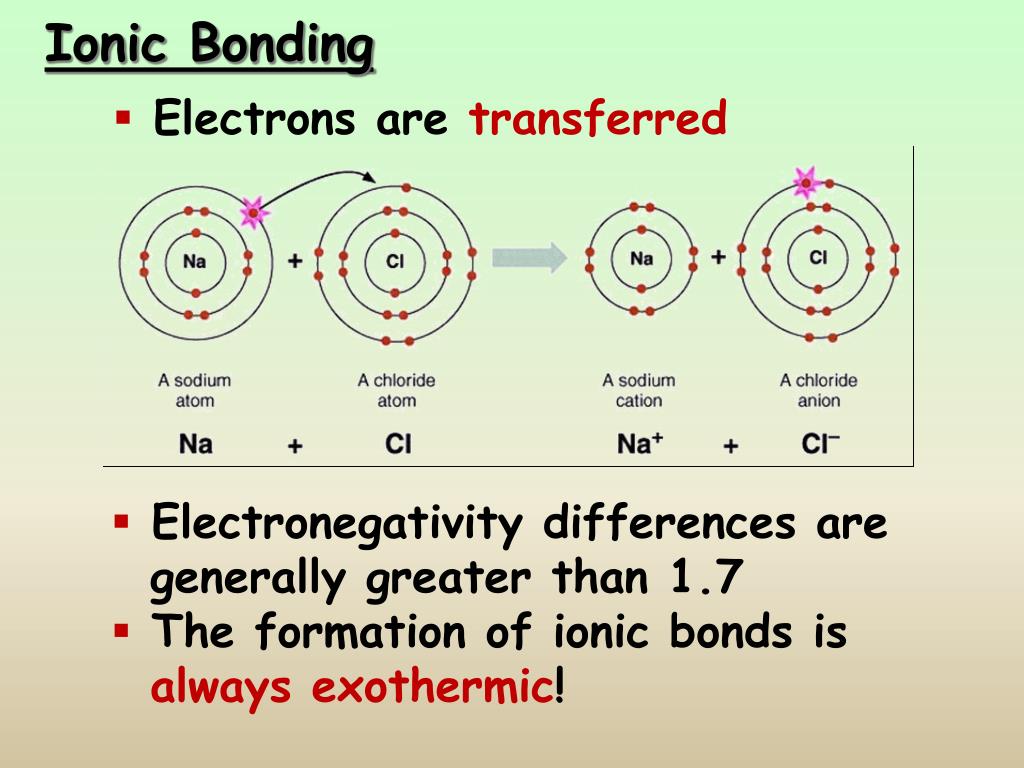
What Does Low Ionic Strength Mean . ionic strength refers to the measure of the total concentration of ions in a solution, calculated based on the molar. for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic strength is. a bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is. the ionic strength of a solution is a quantitative way of expressing the total electrolyte concentration of a solution. in fact, many studies have examined the effect of, or dependence on, not only t, p, or ph, but also ionic strength. in this section, we will examine terms such as 'activity,' define the reference state for quantifying activity, and then.
from mungfali.com
for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic strength is. in this section, we will examine terms such as 'activity,' define the reference state for quantifying activity, and then. the ionic strength of a solution is a quantitative way of expressing the total electrolyte concentration of a solution. a bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is. ionic strength refers to the measure of the total concentration of ions in a solution, calculated based on the molar. in fact, many studies have examined the effect of, or dependence on, not only t, p, or ph, but also ionic strength.
Ppt Naming Ionic And Covalent Compounds Powerpoint Presentation, Free A12
What Does Low Ionic Strength Mean for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic strength is. a bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is. for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic strength is. the ionic strength of a solution is a quantitative way of expressing the total electrolyte concentration of a solution. in this section, we will examine terms such as 'activity,' define the reference state for quantifying activity, and then. in fact, many studies have examined the effect of, or dependence on, not only t, p, or ph, but also ionic strength. ionic strength refers to the measure of the total concentration of ions in a solution, calculated based on the molar.
From www.rapidlabs.co.uk
Low Ionic Strength Solution (LISS) + Additives Rapid Labs What Does Low Ionic Strength Mean the ionic strength of a solution is a quantitative way of expressing the total electrolyte concentration of a solution. for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic strength is. a bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is. . What Does Low Ionic Strength Mean.
From www.mrbarnestc.com
Ionic Equations Mr Barnes Teaches Chemistry What Does Low Ionic Strength Mean for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic strength is. in this section, we will examine terms such as 'activity,' define the reference state for quantifying activity, and then. ionic strength refers to the measure of the total concentration of ions in a solution, calculated based on the molar.. What Does Low Ionic Strength Mean.
From www.researchgate.net
Low ionic strength experiments in water presaturated with calcite and What Does Low Ionic Strength Mean a bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is. the ionic strength of a solution is a quantitative way of expressing the total electrolyte concentration of a solution. in fact, many studies have examined the effect of, or dependence on, not only t, p, or ph, but. What Does Low Ionic Strength Mean.
From www.slideserve.com
PPT ACTIVITY PowerPoint Presentation, free download ID6741459 What Does Low Ionic Strength Mean ionic strength refers to the measure of the total concentration of ions in a solution, calculated based on the molar. in fact, many studies have examined the effect of, or dependence on, not only t, p, or ph, but also ionic strength. for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the. What Does Low Ionic Strength Mean.
From sciencenotes.org
What Is Ionization Energy? Definition and Trend What Does Low Ionic Strength Mean in fact, many studies have examined the effect of, or dependence on, not only t, p, or ph, but also ionic strength. in this section, we will examine terms such as 'activity,' define the reference state for quantifying activity, and then. ionic strength refers to the measure of the total concentration of ions in a solution, calculated. What Does Low Ionic Strength Mean.
From sciencenotes.org
Ionic Bond Definition and Examples What Does Low Ionic Strength Mean for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic strength is. ionic strength refers to the measure of the total concentration of ions in a solution, calculated based on the molar. in fact, many studies have examined the effect of, or dependence on, not only t, p, or ph, but. What Does Low Ionic Strength Mean.
From www.masterorganicchemistry.com
Ionic and Covalent Bonding Master Organic Chemistry What Does Low Ionic Strength Mean the ionic strength of a solution is a quantitative way of expressing the total electrolyte concentration of a solution. a bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is. for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic strength is. . What Does Low Ionic Strength Mean.
From tuitiontube.com
Ionic Bond and Ionic Bond Formation, Definition, Properties in What Does Low Ionic Strength Mean ionic strength refers to the measure of the total concentration of ions in a solution, calculated based on the molar. in fact, many studies have examined the effect of, or dependence on, not only t, p, or ph, but also ionic strength. a bond’s strength describes how strongly each atom is joined to another atom, and therefore. What Does Low Ionic Strength Mean.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM PowerPoint Presentation, free download ID What Does Low Ionic Strength Mean for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic strength is. a bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is. ionic strength refers to the measure of the total concentration of ions in a solution, calculated based on the molar.. What Does Low Ionic Strength Mean.
From en.wikipedia.org
Ionic bonding Wikipedia What Does Low Ionic Strength Mean in this section, we will examine terms such as 'activity,' define the reference state for quantifying activity, and then. for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic strength is. in fact, many studies have examined the effect of, or dependence on, not only t, p, or ph, but also. What Does Low Ionic Strength Mean.
From www.researchgate.net
Compositions of Low Ionic Strength Reference Waters Used in Download What Does Low Ionic Strength Mean the ionic strength of a solution is a quantitative way of expressing the total electrolyte concentration of a solution. for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic strength is. in fact, many studies have examined the effect of, or dependence on, not only t, p, or ph, but also. What Does Low Ionic Strength Mean.
From www.researchgate.net
Ionic composition, total dissolved solids (TDS) and ionic strength of What Does Low Ionic Strength Mean in this section, we will examine terms such as 'activity,' define the reference state for quantifying activity, and then. the ionic strength of a solution is a quantitative way of expressing the total electrolyte concentration of a solution. for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic strength is. . What Does Low Ionic Strength Mean.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts What Does Low Ionic Strength Mean the ionic strength of a solution is a quantitative way of expressing the total electrolyte concentration of a solution. a bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is. for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic strength is. . What Does Low Ionic Strength Mean.
From www.researchgate.net
a At low ionic strength the catch bond formed between intact HMM and What Does Low Ionic Strength Mean ionic strength refers to the measure of the total concentration of ions in a solution, calculated based on the molar. the ionic strength of a solution is a quantitative way of expressing the total electrolyte concentration of a solution. in fact, many studies have examined the effect of, or dependence on, not only t, p, or ph,. What Does Low Ionic Strength Mean.
From www.researchgate.net
Relationship between the molecular force and ionic strength. Download What Does Low Ionic Strength Mean in this section, we will examine terms such as 'activity,' define the reference state for quantifying activity, and then. a bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is. ionic strength refers to the measure of the total concentration of ions in a solution, calculated based on the. What Does Low Ionic Strength Mean.
From mungfali.com
Ppt Naming Ionic And Covalent Compounds Powerpoint Presentation, Free A12 What Does Low Ionic Strength Mean the ionic strength of a solution is a quantitative way of expressing the total electrolyte concentration of a solution. in fact, many studies have examined the effect of, or dependence on, not only t, p, or ph, but also ionic strength. for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic. What Does Low Ionic Strength Mean.
From www.youtube.com
IONIC STRENGTH DEFINATION FORMULA basic introduction YouTube What Does Low Ionic Strength Mean a bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is. ionic strength refers to the measure of the total concentration of ions in a solution, calculated based on the molar. the ionic strength of a solution is a quantitative way of expressing the total electrolyte concentration of a. What Does Low Ionic Strength Mean.
From www.youtube.com
Theory of Thermodynamic Activity and Ionic Strength YouTube What Does Low Ionic Strength Mean in fact, many studies have examined the effect of, or dependence on, not only t, p, or ph, but also ionic strength. for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic strength is. the ionic strength of a solution is a quantitative way of expressing the total electrolyte concentration of. What Does Low Ionic Strength Mean.
From www.slideserve.com
PPT Aqueous Geochemistry PowerPoint Presentation, free download ID What Does Low Ionic Strength Mean a bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is. for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic strength is. in fact, many studies have examined the effect of, or dependence on, not only t, p, or ph, but also. What Does Low Ionic Strength Mean.
From shareeducatonideas.com
What Is An Ionic Compound? Formula and Defination What Does Low Ionic Strength Mean the ionic strength of a solution is a quantitative way of expressing the total electrolyte concentration of a solution. in fact, many studies have examined the effect of, or dependence on, not only t, p, or ph, but also ionic strength. for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic. What Does Low Ionic Strength Mean.
From www.rheosense.com
Ionic Strength & Viscosity Addition of Neutral Salts to Control What Does Low Ionic Strength Mean in this section, we will examine terms such as 'activity,' define the reference state for quantifying activity, and then. for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic strength is. in fact, many studies have examined the effect of, or dependence on, not only t, p, or ph, but also. What Does Low Ionic Strength Mean.
From chem.libretexts.org
Chapter 4.1 Ionic Bonding Chemistry LibreTexts What Does Low Ionic Strength Mean the ionic strength of a solution is a quantitative way of expressing the total electrolyte concentration of a solution. a bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is. for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic strength is. . What Does Low Ionic Strength Mean.
From www.youtube.com
Ionic Strength Introduction YouTube What Does Low Ionic Strength Mean a bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is. for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic strength is. in this section, we will examine terms such as 'activity,' define the reference state for quantifying activity, and then. . What Does Low Ionic Strength Mean.
From www.slideserve.com
PPT Sensitization and Agglutination PowerPoint Presentation ID3923883 What Does Low Ionic Strength Mean ionic strength refers to the measure of the total concentration of ions in a solution, calculated based on the molar. in fact, many studies have examined the effect of, or dependence on, not only t, p, or ph, but also ionic strength. a bond’s strength describes how strongly each atom is joined to another atom, and therefore. What Does Low Ionic Strength Mean.
From courses.lumenlearning.com
Strengths of Ionic and Covalent Bonds Chemistry What Does Low Ionic Strength Mean for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic strength is. a bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is. in this section, we will examine terms such as 'activity,' define the reference state for quantifying activity, and then. . What Does Low Ionic Strength Mean.
From www.youtube.com
Ionic strength YouTube What Does Low Ionic Strength Mean ionic strength refers to the measure of the total concentration of ions in a solution, calculated based on the molar. for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic strength is. the ionic strength of a solution is a quantitative way of expressing the total electrolyte concentration of a solution.. What Does Low Ionic Strength Mean.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica What Does Low Ionic Strength Mean in this section, we will examine terms such as 'activity,' define the reference state for quantifying activity, and then. for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic strength is. a bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is. . What Does Low Ionic Strength Mean.
From www.researchgate.net
Calculated ionic strength for three ranges (low, typical and high What Does Low Ionic Strength Mean for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic strength is. in fact, many studies have examined the effect of, or dependence on, not only t, p, or ph, but also ionic strength. the ionic strength of a solution is a quantitative way of expressing the total electrolyte concentration of. What Does Low Ionic Strength Mean.
From www.slideserve.com
PPT CHEMISTRY 59320 ANALYTICAL CHEMISTRY Fall 2010 PowerPoint What Does Low Ionic Strength Mean a bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is. in fact, many studies have examined the effect of, or dependence on, not only t, p, or ph, but also ionic strength. in this section, we will examine terms such as 'activity,' define the reference state for quantifying. What Does Low Ionic Strength Mean.
From skrooll.com.ph
LOW IONIC STRENGTH SOLUTION (LISS), IMMUADD, IMMUCOR What Does Low Ionic Strength Mean a bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is. ionic strength refers to the measure of the total concentration of ions in a solution, calculated based on the molar. the ionic strength of a solution is a quantitative way of expressing the total electrolyte concentration of a. What Does Low Ionic Strength Mean.
From www.slideserve.com
PPT Solubility of Ionic Compounds PowerPoint Presentation, free What Does Low Ionic Strength Mean in this section, we will examine terms such as 'activity,' define the reference state for quantifying activity, and then. ionic strength refers to the measure of the total concentration of ions in a solution, calculated based on the molar. for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic strength is.. What Does Low Ionic Strength Mean.
From www.researchgate.net
At low ionic strength (50 mM NaCl), topologically closed DNA What Does Low Ionic Strength Mean ionic strength refers to the measure of the total concentration of ions in a solution, calculated based on the molar. in fact, many studies have examined the effect of, or dependence on, not only t, p, or ph, but also ionic strength. the ionic strength of a solution is a quantitative way of expressing the total electrolyte. What Does Low Ionic Strength Mean.
From study.com
Ionic Compound Formation, Properties & Examples Lesson What Does Low Ionic Strength Mean in fact, many studies have examined the effect of, or dependence on, not only t, p, or ph, but also ionic strength. in this section, we will examine terms such as 'activity,' define the reference state for quantifying activity, and then. for a salt such as $\ce{nacl}$ comprising a monovalent cation and a monovalent anion, the ionic. What Does Low Ionic Strength Mean.
From brainly.in
what is the formula for ionic strength Brainly.in What Does Low Ionic Strength Mean in fact, many studies have examined the effect of, or dependence on, not only t, p, or ph, but also ionic strength. ionic strength refers to the measure of the total concentration of ions in a solution, calculated based on the molar. a bond’s strength describes how strongly each atom is joined to another atom, and therefore. What Does Low Ionic Strength Mean.
From www.researchgate.net
A schematic of a calcite surface in low and high ionic strength What Does Low Ionic Strength Mean in this section, we will examine terms such as 'activity,' define the reference state for quantifying activity, and then. in fact, many studies have examined the effect of, or dependence on, not only t, p, or ph, but also ionic strength. a bond’s strength describes how strongly each atom is joined to another atom, and therefore how. What Does Low Ionic Strength Mean.