Israel Medical Device Registration Requirements . As reported by emergo by ul (august 2023), the ministry of health of israel issued several reforms (link in hebrew) to streamline. Medical devices, including biologics that fall under a biologics certificate (bk) process at the usa food and drug administration (fda) center. Gaining access to the israeli medical device market—one of the largest in the middle east—requires registering your device with. In israel, the regulatory body that is responsible for medical device registration, submissions, supervision, and control over the manufacture, assembly, sterilization, import, and. Under the new regulation, which came into force in 2012, all medical devices manufactured or marketed in israel must be.
from www.linkedin.com
Under the new regulation, which came into force in 2012, all medical devices manufactured or marketed in israel must be. Gaining access to the israeli medical device market—one of the largest in the middle east—requires registering your device with. In israel, the regulatory body that is responsible for medical device registration, submissions, supervision, and control over the manufacture, assembly, sterilization, import, and. As reported by emergo by ul (august 2023), the ministry of health of israel issued several reforms (link in hebrew) to streamline. Medical devices, including biologics that fall under a biologics certificate (bk) process at the usa food and drug administration (fda) center.
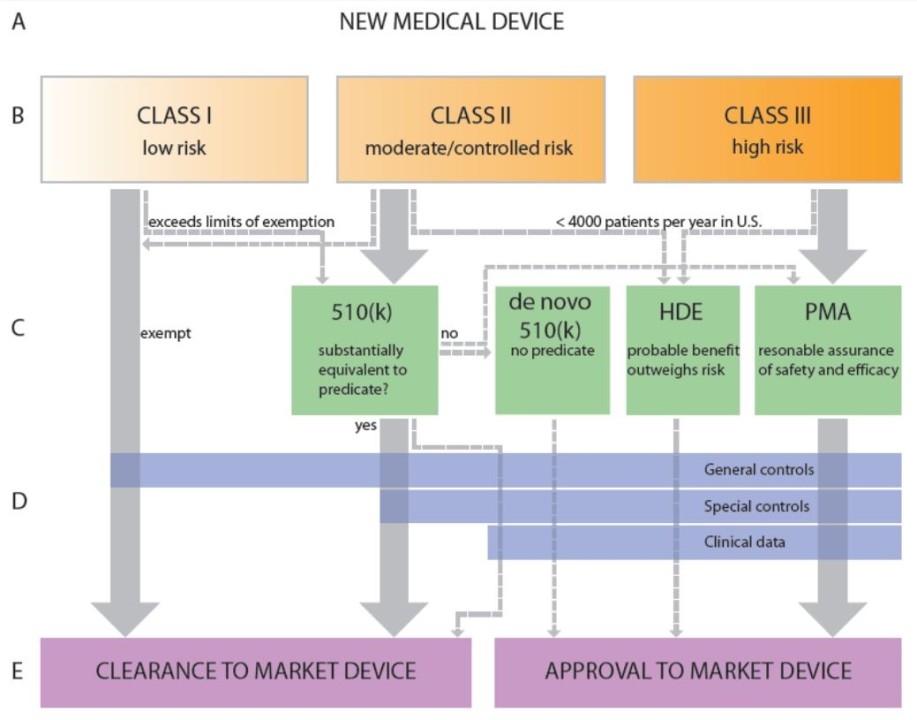
FDA Regulatory Pathways for Medical Devices
Israel Medical Device Registration Requirements Under the new regulation, which came into force in 2012, all medical devices manufactured or marketed in israel must be. In israel, the regulatory body that is responsible for medical device registration, submissions, supervision, and control over the manufacture, assembly, sterilization, import, and. As reported by emergo by ul (august 2023), the ministry of health of israel issued several reforms (link in hebrew) to streamline. Gaining access to the israeli medical device market—one of the largest in the middle east—requires registering your device with. Medical devices, including biologics that fall under a biologics certificate (bk) process at the usa food and drug administration (fda) center. Under the new regulation, which came into force in 2012, all medical devices manufactured or marketed in israel must be.
From medicaldevices.freyrsolutions.com
Medical Device Registration in Israel Freyr Medical Devices Israel Medical Device Registration Requirements Gaining access to the israeli medical device market—one of the largest in the middle east—requires registering your device with. As reported by emergo by ul (august 2023), the ministry of health of israel issued several reforms (link in hebrew) to streamline. In israel, the regulatory body that is responsible for medical device registration, submissions, supervision, and control over the manufacture,. Israel Medical Device Registration Requirements.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Israel Medical Device Registration Requirements As reported by emergo by ul (august 2023), the ministry of health of israel issued several reforms (link in hebrew) to streamline. Medical devices, including biologics that fall under a biologics certificate (bk) process at the usa food and drug administration (fda) center. Under the new regulation, which came into force in 2012, all medical devices manufactured or marketed in. Israel Medical Device Registration Requirements.
From www.youtube.com
Registration Certificate for the Sale or Distribution of Medical Israel Medical Device Registration Requirements In israel, the regulatory body that is responsible for medical device registration, submissions, supervision, and control over the manufacture, assembly, sterilization, import, and. Under the new regulation, which came into force in 2012, all medical devices manufactured or marketed in israel must be. Medical devices, including biologics that fall under a biologics certificate (bk) process at the usa food and. Israel Medical Device Registration Requirements.
From www.slideshare.net
Israel medical devices industry Market Overview Israel Medical Device Registration Requirements Gaining access to the israeli medical device market—one of the largest in the middle east—requires registering your device with. Under the new regulation, which came into force in 2012, all medical devices manufactured or marketed in israel must be. In israel, the regulatory body that is responsible for medical device registration, submissions, supervision, and control over the manufacture, assembly, sterilization,. Israel Medical Device Registration Requirements.
From sveforum.org
Israel Group Medical Plan Insurance July 2023 Silicon Valley Israel Medical Device Registration Requirements Medical devices, including biologics that fall under a biologics certificate (bk) process at the usa food and drug administration (fda) center. As reported by emergo by ul (august 2023), the ministry of health of israel issued several reforms (link in hebrew) to streamline. In israel, the regulatory body that is responsible for medical device registration, submissions, supervision, and control over. Israel Medical Device Registration Requirements.
From www.orielstat.com
Class 1 Medical Device Requirements Oriel STAT A MATRIX Israel Medical Device Registration Requirements As reported by emergo by ul (august 2023), the ministry of health of israel issued several reforms (link in hebrew) to streamline. Gaining access to the israeli medical device market—one of the largest in the middle east—requires registering your device with. Medical devices, including biologics that fall under a biologics certificate (bk) process at the usa food and drug administration. Israel Medical Device Registration Requirements.
From operonstrategist.com
5 Tips for Successful Medical Device Registration Across Global Markets Israel Medical Device Registration Requirements In israel, the regulatory body that is responsible for medical device registration, submissions, supervision, and control over the manufacture, assembly, sterilization, import, and. Under the new regulation, which came into force in 2012, all medical devices manufactured or marketed in israel must be. Medical devices, including biologics that fall under a biologics certificate (bk) process at the usa food and. Israel Medical Device Registration Requirements.
From learn.marsdd.com
Medical device submissions Placing a medical device on the market Israel Medical Device Registration Requirements As reported by emergo by ul (august 2023), the ministry of health of israel issued several reforms (link in hebrew) to streamline. Under the new regulation, which came into force in 2012, all medical devices manufactured or marketed in israel must be. In israel, the regulatory body that is responsible for medical device registration, submissions, supervision, and control over the. Israel Medical Device Registration Requirements.
From www.alfusaic.net
Israel Healthcare 101 — Al Fusaic Israel Medical Device Registration Requirements As reported by emergo by ul (august 2023), the ministry of health of israel issued several reforms (link in hebrew) to streamline. Medical devices, including biologics that fall under a biologics certificate (bk) process at the usa food and drug administration (fda) center. Under the new regulation, which came into force in 2012, all medical devices manufactured or marketed in. Israel Medical Device Registration Requirements.
From www.medica-tradefair.com
Israeli medical devices showcase digital innovations at MEDICA Israel Medical Device Registration Requirements As reported by emergo by ul (august 2023), the ministry of health of israel issued several reforms (link in hebrew) to streamline. Gaining access to the israeli medical device market—one of the largest in the middle east—requires registering your device with. Medical devices, including biologics that fall under a biologics certificate (bk) process at the usa food and drug administration. Israel Medical Device Registration Requirements.
From eranyona.com
SaMD, IVD, and EHealth Services Registration In Israel guideline Israel Medical Device Registration Requirements As reported by emergo by ul (august 2023), the ministry of health of israel issued several reforms (link in hebrew) to streamline. In israel, the regulatory body that is responsible for medical device registration, submissions, supervision, and control over the manufacture, assembly, sterilization, import, and. Under the new regulation, which came into force in 2012, all medical devices manufactured or. Israel Medical Device Registration Requirements.
From pharmaknowl.com
SFDA Medical Device Registration in Saudi Arabia (2023 Guide Israel Medical Device Registration Requirements Gaining access to the israeli medical device market—one of the largest in the middle east—requires registering your device with. In israel, the regulatory body that is responsible for medical device registration, submissions, supervision, and control over the manufacture, assembly, sterilization, import, and. As reported by emergo by ul (august 2023), the ministry of health of israel issued several reforms (link. Israel Medical Device Registration Requirements.
From www.mpo-mag.com
International Law Medical Product Outsourcing Israel Medical Device Registration Requirements Medical devices, including biologics that fall under a biologics certificate (bk) process at the usa food and drug administration (fda) center. Gaining access to the israeli medical device market—one of the largest in the middle east—requires registering your device with. Under the new regulation, which came into force in 2012, all medical devices manufactured or marketed in israel must be.. Israel Medical Device Registration Requirements.
From www.linkedin.com
FDA Regulatory Pathways for Medical Devices Israel Medical Device Registration Requirements Under the new regulation, which came into force in 2012, all medical devices manufactured or marketed in israel must be. Medical devices, including biologics that fall under a biologics certificate (bk) process at the usa food and drug administration (fda) center. In israel, the regulatory body that is responsible for medical device registration, submissions, supervision, and control over the manufacture,. Israel Medical Device Registration Requirements.
From sync.co.il
Virtual Meeting Of The Israeli Medical Devices Community MDI Virtual Israel Medical Device Registration Requirements As reported by emergo by ul (august 2023), the ministry of health of israel issued several reforms (link in hebrew) to streamline. Medical devices, including biologics that fall under a biologics certificate (bk) process at the usa food and drug administration (fda) center. Gaining access to the israeli medical device market—one of the largest in the middle east—requires registering your. Israel Medical Device Registration Requirements.
From eranyona.com
Medical device registration and submission in Israel BioChem Israel Medical Device Registration Requirements Gaining access to the israeli medical device market—one of the largest in the middle east—requires registering your device with. As reported by emergo by ul (august 2023), the ministry of health of israel issued several reforms (link in hebrew) to streamline. Under the new regulation, which came into force in 2012, all medical devices manufactured or marketed in israel must. Israel Medical Device Registration Requirements.
From www.emergobyul.com
Israel Medical Device Registration and Approval AMAR Israel Medical Device Registration Requirements In israel, the regulatory body that is responsible for medical device registration, submissions, supervision, and control over the manufacture, assembly, sterilization, import, and. Gaining access to the israeli medical device market—one of the largest in the middle east—requires registering your device with. Under the new regulation, which came into force in 2012, all medical devices manufactured or marketed in israel. Israel Medical Device Registration Requirements.
From coat-x.com
COATX gets its ISO13485 Medical Device certificate COATX Israel Medical Device Registration Requirements In israel, the regulatory body that is responsible for medical device registration, submissions, supervision, and control over the manufacture, assembly, sterilization, import, and. Gaining access to the israeli medical device market—one of the largest in the middle east—requires registering your device with. As reported by emergo by ul (august 2023), the ministry of health of israel issued several reforms (link. Israel Medical Device Registration Requirements.
From www.youtube.com
Israeli Medical Technologies YouTube Israel Medical Device Registration Requirements Under the new regulation, which came into force in 2012, all medical devices manufactured or marketed in israel must be. Gaining access to the israeli medical device market—one of the largest in the middle east—requires registering your device with. As reported by emergo by ul (august 2023), the ministry of health of israel issued several reforms (link in hebrew) to. Israel Medical Device Registration Requirements.
From www.complianceandrisks.com
Medical Device Regulation Israel Compliance & Risks Israel Medical Device Registration Requirements Under the new regulation, which came into force in 2012, all medical devices manufactured or marketed in israel must be. In israel, the regulatory body that is responsible for medical device registration, submissions, supervision, and control over the manufacture, assembly, sterilization, import, and. Medical devices, including biologics that fall under a biologics certificate (bk) process at the usa food and. Israel Medical Device Registration Requirements.
From www.access.fda.gov
Register a New Medical Device Facility StepbyStep Instructions Israel Medical Device Registration Requirements Under the new regulation, which came into force in 2012, all medical devices manufactured or marketed in israel must be. As reported by emergo by ul (august 2023), the ministry of health of israel issued several reforms (link in hebrew) to streamline. Medical devices, including biologics that fall under a biologics certificate (bk) process at the usa food and drug. Israel Medical Device Registration Requirements.
From www.access.fda.gov
Register a New Medical Device Facility StepbyStep Instructions Israel Medical Device Registration Requirements Medical devices, including biologics that fall under a biologics certificate (bk) process at the usa food and drug administration (fda) center. As reported by emergo by ul (august 2023), the ministry of health of israel issued several reforms (link in hebrew) to streamline. In israel, the regulatory body that is responsible for medical device registration, submissions, supervision, and control over. Israel Medical Device Registration Requirements.
From template.mapadapalavra.ba.gov.br
Medical Device Regulatory Strategy Template Israel Medical Device Registration Requirements In israel, the regulatory body that is responsible for medical device registration, submissions, supervision, and control over the manufacture, assembly, sterilization, import, and. Under the new regulation, which came into force in 2012, all medical devices manufactured or marketed in israel must be. Gaining access to the israeli medical device market—one of the largest in the middle east—requires registering your. Israel Medical Device Registration Requirements.
From enika.com
ENIKA SURGICAL MFG CO. Israel Medical Device Registration Requirements Under the new regulation, which came into force in 2012, all medical devices manufactured or marketed in israel must be. In israel, the regulatory body that is responsible for medical device registration, submissions, supervision, and control over the manufacture, assembly, sterilization, import, and. Medical devices, including biologics that fall under a biologics certificate (bk) process at the usa food and. Israel Medical Device Registration Requirements.
From fyolyhfuf.blob.core.windows.net
Fda Device Labeling Requirements at Paul Cangelosi blog Israel Medical Device Registration Requirements Under the new regulation, which came into force in 2012, all medical devices manufactured or marketed in israel must be. Medical devices, including biologics that fall under a biologics certificate (bk) process at the usa food and drug administration (fda) center. As reported by emergo by ul (august 2023), the ministry of health of israel issued several reforms (link in. Israel Medical Device Registration Requirements.
From eranyona.com
Medical Device import/export and registration regulatory requirements Israel Medical Device Registration Requirements Gaining access to the israeli medical device market—one of the largest in the middle east—requires registering your device with. Medical devices, including biologics that fall under a biologics certificate (bk) process at the usa food and drug administration (fda) center. In israel, the regulatory body that is responsible for medical device registration, submissions, supervision, and control over the manufacture, assembly,. Israel Medical Device Registration Requirements.
From fyolyhfuf.blob.core.windows.net
Fda Device Labeling Requirements at Paul Cangelosi blog Israel Medical Device Registration Requirements Gaining access to the israeli medical device market—one of the largest in the middle east—requires registering your device with. In israel, the regulatory body that is responsible for medical device registration, submissions, supervision, and control over the manufacture, assembly, sterilization, import, and. Medical devices, including biologics that fall under a biologics certificate (bk) process at the usa food and drug. Israel Medical Device Registration Requirements.
From www.slideshare.net
Europe CE Marking for medical devices under new MDR Israel Medical Device Registration Requirements Gaining access to the israeli medical device market—one of the largest in the middle east—requires registering your device with. Under the new regulation, which came into force in 2012, all medical devices manufactured or marketed in israel must be. In israel, the regulatory body that is responsible for medical device registration, submissions, supervision, and control over the manufacture, assembly, sterilization,. Israel Medical Device Registration Requirements.
From joli-360.com
Regulation and Certification JÓLI360 E.S.I Novel Israel Medical Device Registration Requirements Medical devices, including biologics that fall under a biologics certificate (bk) process at the usa food and drug administration (fda) center. Gaining access to the israeli medical device market—one of the largest in the middle east—requires registering your device with. Under the new regulation, which came into force in 2012, all medical devices manufactured or marketed in israel must be.. Israel Medical Device Registration Requirements.
From arazygroup.com
Israel Arazy Group Israel Medical Device Registration Requirements Medical devices, including biologics that fall under a biologics certificate (bk) process at the usa food and drug administration (fda) center. As reported by emergo by ul (august 2023), the ministry of health of israel issued several reforms (link in hebrew) to streamline. In israel, the regulatory body that is responsible for medical device registration, submissions, supervision, and control over. Israel Medical Device Registration Requirements.
From www.freyrsolutions.com
UKCA Marking Requirements for Medical Devices Freyr Global Israel Medical Device Registration Requirements Medical devices, including biologics that fall under a biologics certificate (bk) process at the usa food and drug administration (fda) center. As reported by emergo by ul (august 2023), the ministry of health of israel issued several reforms (link in hebrew) to streamline. In israel, the regulatory body that is responsible for medical device registration, submissions, supervision, and control over. Israel Medical Device Registration Requirements.
From medicaldeviceacademy.com
Private Labeled Devices with FDA Approval Medical Device Academy Israel Medical Device Registration Requirements Under the new regulation, which came into force in 2012, all medical devices manufactured or marketed in israel must be. As reported by emergo by ul (august 2023), the ministry of health of israel issued several reforms (link in hebrew) to streamline. Gaining access to the israeli medical device market—one of the largest in the middle east—requires registering your device. Israel Medical Device Registration Requirements.
From www.globaldata.com
Israel Healthcare (Pharma and Medical Devices) Market Analysis Israel Medical Device Registration Requirements In israel, the regulatory body that is responsible for medical device registration, submissions, supervision, and control over the manufacture, assembly, sterilization, import, and. As reported by emergo by ul (august 2023), the ministry of health of israel issued several reforms (link in hebrew) to streamline. Under the new regulation, which came into force in 2012, all medical devices manufactured or. Israel Medical Device Registration Requirements.
From www.medica-tradefair.com
Israeli medical devices showcase digital innovations at MEDICA Israel Medical Device Registration Requirements In israel, the regulatory body that is responsible for medical device registration, submissions, supervision, and control over the manufacture, assembly, sterilization, import, and. Under the new regulation, which came into force in 2012, all medical devices manufactured or marketed in israel must be. Gaining access to the israeli medical device market—one of the largest in the middle east—requires registering your. Israel Medical Device Registration Requirements.
From operonstrategist.com
Comprehensive Guide to Medical Device Registration in LATAM Countries Israel Medical Device Registration Requirements Under the new regulation, which came into force in 2012, all medical devices manufactured or marketed in israel must be. As reported by emergo by ul (august 2023), the ministry of health of israel issued several reforms (link in hebrew) to streamline. Gaining access to the israeli medical device market—one of the largest in the middle east—requires registering your device. Israel Medical Device Registration Requirements.