Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers . Food and drug administration (fda). cdrh guidance on medical device patient labeling; Final guidance for industry and fda reviewers. guidance on medical device patient labeling final guidance for industry and fda staffapril 2001. this guidance is designed to help assure safe and effective use of medical devices through medical device patient labeling that informs patients or their lay caregivers about proper use, risks, and. Final guidance for industry and fda staff. Fda guidance on medical device patient labeling; guidance on medical device patient labeling: for information on developing patient labeling for medical devices, including in vitro diagnostic products, please see 21 cfr parts 801 and 809 and the fda web page at. Download the final guidance document.
from www.slideshare.net
guidance on medical device patient labeling final guidance for industry and fda staffapril 2001. guidance on medical device patient labeling: Fda guidance on medical device patient labeling; Final guidance for industry and fda reviewers. this guidance is designed to help assure safe and effective use of medical devices through medical device patient labeling that informs patients or their lay caregivers about proper use, risks, and. cdrh guidance on medical device patient labeling; Food and drug administration (fda). Final guidance for industry and fda staff. for information on developing patient labeling for medical devices, including in vitro diagnostic products, please see 21 cfr parts 801 and 809 and the fda web page at. Download the final guidance document.
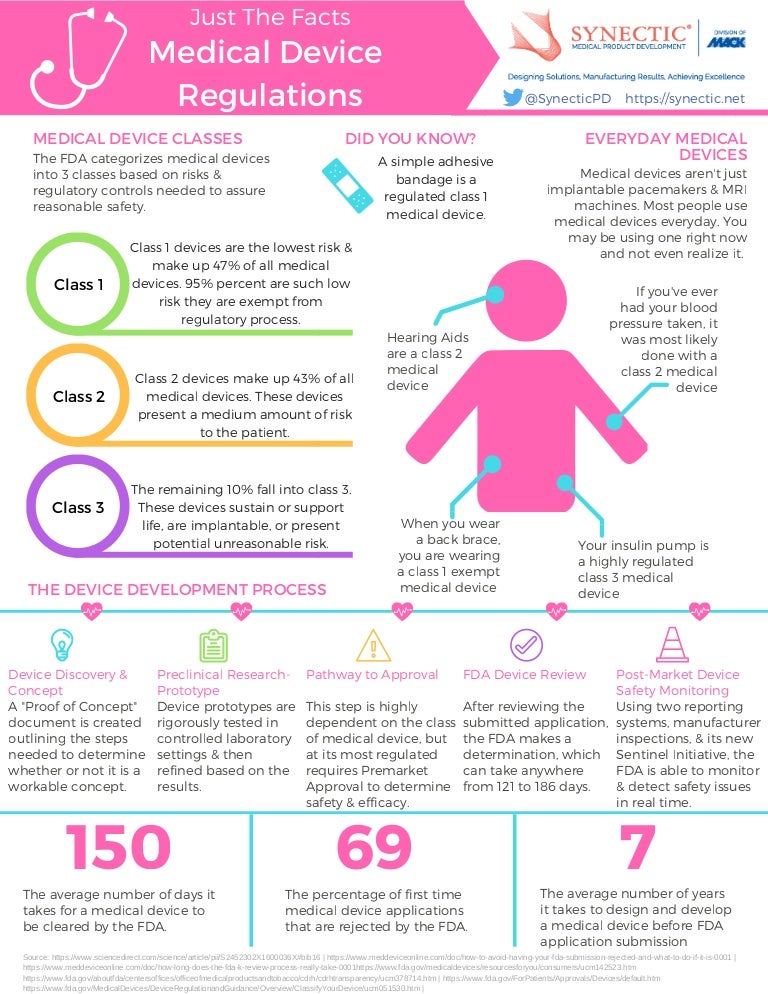
Medical Device FDA Regulations and Classifications infographic
Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers Fda guidance on medical device patient labeling; Download the final guidance document. Fda guidance on medical device patient labeling; this guidance is designed to help assure safe and effective use of medical devices through medical device patient labeling that informs patients or their lay caregivers about proper use, risks, and. Final guidance for industry and fda reviewers. Final guidance for industry and fda staff. Food and drug administration (fda). guidance on medical device patient labeling: for information on developing patient labeling for medical devices, including in vitro diagnostic products, please see 21 cfr parts 801 and 809 and the fda web page at. cdrh guidance on medical device patient labeling; guidance on medical device patient labeling final guidance for industry and fda staffapril 2001.
From www.regdesk.co
FDA Guidance on Medical Device Patient Labeling Overview RegDesk Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers Final guidance for industry and fda reviewers. Fda guidance on medical device patient labeling; guidance on medical device patient labeling: this guidance is designed to help assure safe and effective use of medical devices through medical device patient labeling that informs patients or their lay caregivers about proper use, risks, and. for information on developing patient labeling. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From www.regdesk.co
EFDA Guidance on Medical Device Labeling Location and Content RegDesk Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers Final guidance for industry and fda reviewers. Final guidance for industry and fda staff. Download the final guidance document. guidance on medical device patient labeling: Food and drug administration (fda). this guidance is designed to help assure safe and effective use of medical devices through medical device patient labeling that informs patients or their lay caregivers about proper. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From www.regdesk.co
EFDA Guidance on Medical Device Labeling Location and Content RegDesk Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers Food and drug administration (fda). this guidance is designed to help assure safe and effective use of medical devices through medical device patient labeling that informs patients or their lay caregivers about proper use, risks, and. Final guidance for industry and fda reviewers. Final guidance for industry and fda staff. cdrh guidance on medical device patient labeling; . Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers Food and drug administration (fda). this guidance is designed to help assure safe and effective use of medical devices through medical device patient labeling that informs patients or their lay caregivers about proper use, risks, and. Final guidance for industry and fda staff. Fda guidance on medical device patient labeling; guidance on medical device patient labeling: for. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From www.finnegan.com
Final FDA Guidance on Safety Considerations for Medication Container Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers Fda guidance on medical device patient labeling; Food and drug administration (fda). Download the final guidance document. guidance on medical device patient labeling: cdrh guidance on medical device patient labeling; this guidance is designed to help assure safe and effective use of medical devices through medical device patient labeling that informs patients or their lay caregivers about. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From quandarypeak.com
FDA Releases Final Guidance on Clinical Decision Support Software as a Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers Download the final guidance document. Fda guidance on medical device patient labeling; this guidance is designed to help assure safe and effective use of medical devices through medical device patient labeling that informs patients or their lay caregivers about proper use, risks, and. Final guidance for industry and fda reviewers. cdrh guidance on medical device patient labeling; Final. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From barcode-labels.com
Medical Devices Electronic Imaging Materials Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers cdrh guidance on medical device patient labeling; this guidance is designed to help assure safe and effective use of medical devices through medical device patient labeling that informs patients or their lay caregivers about proper use, risks, and. Download the final guidance document. guidance on medical device patient labeling final guidance for industry and fda staffapril 2001.. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From mavink.com
Medical Device Labeling Symbols Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers Final guidance for industry and fda reviewers. cdrh guidance on medical device patient labeling; for information on developing patient labeling for medical devices, including in vitro diagnostic products, please see 21 cfr parts 801 and 809 and the fda web page at. this guidance is designed to help assure safe and effective use of medical devices through. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers cdrh guidance on medical device patient labeling; Final guidance for industry and fda reviewers. this guidance is designed to help assure safe and effective use of medical devices through medical device patient labeling that informs patients or their lay caregivers about proper use, risks, and. Final guidance for industry and fda staff. Food and drug administration (fda). Download. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers Food and drug administration (fda). Fda guidance on medical device patient labeling; guidance on medical device patient labeling final guidance for industry and fda staffapril 2001. for information on developing patient labeling for medical devices, including in vitro diagnostic products, please see 21 cfr parts 801 and 809 and the fda web page at. guidance on medical. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From www.regdesk.co
FDA Guidance on Medical Device Patient Labeling Warnings and Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers guidance on medical device patient labeling: this guidance is designed to help assure safe and effective use of medical devices through medical device patient labeling that informs patients or their lay caregivers about proper use, risks, and. Fda guidance on medical device patient labeling; Food and drug administration (fda). Download the final guidance document. guidance on medical. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers cdrh guidance on medical device patient labeling; Final guidance for industry and fda staff. Fda guidance on medical device patient labeling; Download the final guidance document. Final guidance for industry and fda reviewers. guidance on medical device patient labeling: guidance on medical device patient labeling final guidance for industry and fda staffapril 2001. for information on. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From www.regdesk.co
FDA Guidance on Reprocessing Medical Devices Validation and FDA Review Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers guidance on medical device patient labeling: Fda guidance on medical device patient labeling; cdrh guidance on medical device patient labeling; Final guidance for industry and fda reviewers. Food and drug administration (fda). Final guidance for industry and fda staff. guidance on medical device patient labeling final guidance for industry and fda staffapril 2001. this guidance is. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From www.slideshare.net
Medical Device FDA Regulations and Classifications infographic Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers Final guidance for industry and fda reviewers. Fda guidance on medical device patient labeling; cdrh guidance on medical device patient labeling; for information on developing patient labeling for medical devices, including in vitro diagnostic products, please see 21 cfr parts 801 and 809 and the fda web page at. Download the final guidance document. guidance on medical. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers Final guidance for industry and fda reviewers. Download the final guidance document. Fda guidance on medical device patient labeling; for information on developing patient labeling for medical devices, including in vitro diagnostic products, please see 21 cfr parts 801 and 809 and the fda web page at. Final guidance for industry and fda staff. guidance on medical device. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From www.presentationeze.com
FDA Medical Device Labeling requirements. PresentationEZE Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers for information on developing patient labeling for medical devices, including in vitro diagnostic products, please see 21 cfr parts 801 and 809 and the fda web page at. Final guidance for industry and fda reviewers. Food and drug administration (fda). Download the final guidance document. Fda guidance on medical device patient labeling; guidance on medical device patient labeling:. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From www.youtube.com
15327_FDA Medical Device Regulations Labeling Requirements YouTube Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers for information on developing patient labeling for medical devices, including in vitro diagnostic products, please see 21 cfr parts 801 and 809 and the fda web page at. guidance on medical device patient labeling: guidance on medical device patient labeling final guidance for industry and fda staffapril 2001. Final guidance for industry and fda staff. Fda guidance. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From www.regdesk.co
EFDA Guidance on Medical Device Labeling Overview RegDesk Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers guidance on medical device patient labeling: Final guidance for industry and fda reviewers. for information on developing patient labeling for medical devices, including in vitro diagnostic products, please see 21 cfr parts 801 and 809 and the fda web page at. Food and drug administration (fda). guidance on medical device patient labeling final guidance for industry and. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From www.regdesk.co
FDA Guidance on Medical Device Patient Labeling Readability RegDesk Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers Food and drug administration (fda). Download the final guidance document. Fda guidance on medical device patient labeling; this guidance is designed to help assure safe and effective use of medical devices through medical device patient labeling that informs patients or their lay caregivers about proper use, risks, and. Final guidance for industry and fda staff. guidance on medical. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From www.regdesk.co
FDA Guidance on Development of Medical Device Labeling RegDesk Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers Download the final guidance document. Final guidance for industry and fda reviewers. Food and drug administration (fda). Fda guidance on medical device patient labeling; guidance on medical device patient labeling: Final guidance for industry and fda staff. this guidance is designed to help assure safe and effective use of medical devices through medical device patient labeling that informs. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From docslib.org
Guidance on Medical Device Patient Labeling; Final Guidance for Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers guidance on medical device patient labeling: Final guidance for industry and fda reviewers. Fda guidance on medical device patient labeling; guidance on medical device patient labeling final guidance for industry and fda staffapril 2001. cdrh guidance on medical device patient labeling; Food and drug administration (fda). Final guidance for industry and fda staff. Download the final guidance. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From www.regdesk.co
FDA Guidance on Labeling for In Vitro Diagnostic Devices RegDesk Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers Food and drug administration (fda). for information on developing patient labeling for medical devices, including in vitro diagnostic products, please see 21 cfr parts 801 and 809 and the fda web page at. Final guidance for industry and fda staff. Download the final guidance document. guidance on medical device patient labeling: cdrh guidance on medical device patient. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From mungfali.com
FDA Medical Device Label Symbols Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers Food and drug administration (fda). guidance on medical device patient labeling final guidance for industry and fda staffapril 2001. Download the final guidance document. Fda guidance on medical device patient labeling; for information on developing patient labeling for medical devices, including in vitro diagnostic products, please see 21 cfr parts 801 and 809 and the fda web page. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From www.regdesk.co
FDA Guidance on Medical Device Patient Labeling Readability RegDesk Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers Final guidance for industry and fda staff. for information on developing patient labeling for medical devices, including in vitro diagnostic products, please see 21 cfr parts 801 and 809 and the fda web page at. cdrh guidance on medical device patient labeling; Food and drug administration (fda). Final guidance for industry and fda reviewers. guidance on medical. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From www.fda.gov
Patient Labeling Resources FDA Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers Final guidance for industry and fda staff. Final guidance for industry and fda reviewers. guidance on medical device patient labeling final guidance for industry and fda staffapril 2001. for information on developing patient labeling for medical devices, including in vitro diagnostic products, please see 21 cfr parts 801 and 809 and the fda web page at. Food and. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 55 OFF Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers Final guidance for industry and fda reviewers. Food and drug administration (fda). this guidance is designed to help assure safe and effective use of medical devices through medical device patient labeling that informs patients or their lay caregivers about proper use, risks, and. for information on developing patient labeling for medical devices, including in vitro diagnostic products, please. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From templates.rjuuc.edu.np
Medical Device Label Template Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers Fda guidance on medical device patient labeling; this guidance is designed to help assure safe and effective use of medical devices through medical device patient labeling that informs patients or their lay caregivers about proper use, risks, and. Download the final guidance document. for information on developing patient labeling for medical devices, including in vitro diagnostic products, please. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers guidance on medical device patient labeling: Fda guidance on medical device patient labeling; Final guidance for industry and fda reviewers. Food and drug administration (fda). cdrh guidance on medical device patient labeling; Download the final guidance document. Final guidance for industry and fda staff. guidance on medical device patient labeling final guidance for industry and fda staffapril. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From emmainternational.com
Understanding FDA Guidance Documents Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers cdrh guidance on medical device patient labeling; Download the final guidance document. guidance on medical device patient labeling final guidance for industry and fda staffapril 2001. for information on developing patient labeling for medical devices, including in vitro diagnostic products, please see 21 cfr parts 801 and 809 and the fda web page at. Final guidance for. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From www.regdesk.co
EFDA Guidance on Medical Device Labeling Special Requirements RegDesk Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers for information on developing patient labeling for medical devices, including in vitro diagnostic products, please see 21 cfr parts 801 and 809 and the fda web page at. cdrh guidance on medical device patient labeling; Fda guidance on medical device patient labeling; this guidance is designed to help assure safe and effective use of medical devices through. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From www.regdesk.co
FDA Guidance on Testing and Labelling Medical Devices for Safety in the Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers guidance on medical device patient labeling: Download the final guidance document. Final guidance for industry and fda staff. Food and drug administration (fda). Fda guidance on medical device patient labeling; Final guidance for industry and fda reviewers. cdrh guidance on medical device patient labeling; this guidance is designed to help assure safe and effective use of medical. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From www.regdesk.co
FDA Guidance on Medical Device Reporting Written Procedures, Record Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers this guidance is designed to help assure safe and effective use of medical devices through medical device patient labeling that informs patients or their lay caregivers about proper use, risks, and. Final guidance for industry and fda staff. guidance on medical device patient labeling: guidance on medical device patient labeling final guidance for industry and fda staffapril. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From www.pinterest.com
Infographic on Understanding FDA Device Classes from Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers for information on developing patient labeling for medical devices, including in vitro diagnostic products, please see 21 cfr parts 801 and 809 and the fda web page at. Final guidance for industry and fda staff. Final guidance for industry and fda reviewers. Food and drug administration (fda). Download the final guidance document. this guidance is designed to help. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From www.regdesk.co
FDA Guidance on Medical Device Data Systems and Image Devices RegDesk Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers guidance on medical device patient labeling: Final guidance for industry and fda reviewers. guidance on medical device patient labeling final guidance for industry and fda staffapril 2001. for information on developing patient labeling for medical devices, including in vitro diagnostic products, please see 21 cfr parts 801 and 809 and the fda web page at. cdrh. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.
From www.regdesk.co
FDA Guidance on Medical Device Reporting Specific Issues Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers Final guidance for industry and fda staff. this guidance is designed to help assure safe and effective use of medical devices through medical device patient labeling that informs patients or their lay caregivers about proper use, risks, and. guidance on medical device patient labeling: cdrh guidance on medical device patient labeling; Food and drug administration (fda). Download. Guidance On Medical Device Patient Labeling Final Guidance For Industry And Fda Reviewers.