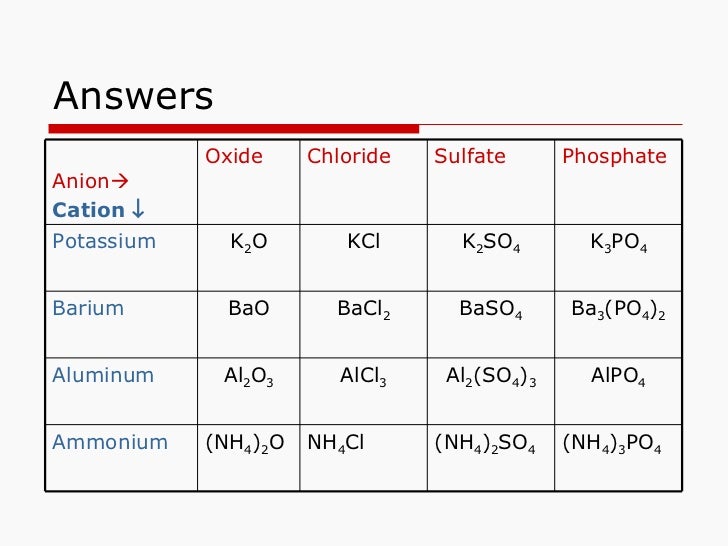
Aluminum Oxide Cation . The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form. Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. An aluminum cation is a positively charged aluminum ion that is part of a cationic aluminum complex used as a polymerization catalyst for. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do.
from www.slideshare.net
Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. An aluminum cation is a positively charged aluminum ion that is part of a cationic aluminum complex used as a polymerization catalyst for. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form.
Ch 8 ionic compounds
Aluminum Oxide Cation For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form. An aluminum cation is a positively charged aluminum ion that is part of a cationic aluminum complex used as a polymerization catalyst for. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three.
From www.coursehero.com
[Solved] Cation Anion Formula Name sodium iodide aluminum bromide Aluminum Oxide Cation Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form. An aluminum cation is a positively charged aluminum ion that is part of a cationic aluminum complex used as a polymerization catalyst for. Aluminium oxide contains oxide ions,. Aluminum Oxide Cation.
From www.slideserve.com
PPT WarmUp PowerPoint Presentation, free download ID3731178 Aluminum Oxide Cation For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. An aluminum cation is a positively charged aluminum ion that is part of a cationic aluminum complex used as a polymerization catalyst for. The lighter group 3a metals (aluminum, galium and indium), along with scandium. Aluminum Oxide Cation.
From www.researchgate.net
Schematic twodimensional representation of aluminum oxide structures Aluminum Oxide Cation Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form. Aluminium oxide contains. Aluminum Oxide Cation.
From www.slideshare.net
Ionic bonding binary Aluminum Oxide Cation The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form. An aluminum cation is a positively charged aluminum ion that is part of a cationic aluminum complex used as a polymerization catalyst for. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two. Aluminum Oxide Cation.
From www.youtube.com
Writing the Formula for Aluminum Oxide YouTube Aluminum Oxide Cation Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+,. Aluminum Oxide Cation.
From brainly.in
explain the formation of aluminium oxide with electronic dot Aluminum Oxide Cation An aluminum cation is a positively charged aluminum ion that is part of a cationic aluminum complex used as a polymerization catalyst for. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. For example, the formula for. Aluminum Oxide Cation.
From www.goodscience.com.au
Determining the Formula for Ionic Compounds Good Science Aluminum Oxide Cation Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. An aluminum cation is a positively charged aluminum ion that is part of a cationic aluminum complex used as a polymerization catalyst for. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every. Aluminum Oxide Cation.
From ar.inspiredpencil.com
Aluminum Oxide Structure Aluminum Oxide Cation Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. An aluminum cation is a positively charged aluminum ion that is part of a cationic aluminum complex used as a polymerization catalyst for. The lighter group 3a metals. Aluminum Oxide Cation.
From www.frontiersin.org
Frontiers Field Strength of NetworkModifying Cation Dictates the Aluminum Oxide Cation Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. An aluminum cation is a positively charged aluminum ion that is part of a cationic aluminum complex used as a polymerization catalyst for. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two. Aluminum Oxide Cation.
From www.slideserve.com
PPT Chemical Formulas, and Nomenclature ch 9 PowerPoint Presentation Aluminum Oxide Cation Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. An aluminum cation is a positively charged aluminum ion that is part of a cationic aluminum complex used as a polymerization catalyst for. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. The lighter group 3a metals. Aluminum Oxide Cation.
From pubs.acs.org
Structural Characterization of NickelDoped Aluminum Oxide Cations by Aluminum Oxide Cation For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3. Aluminum Oxide Cation.
From ar.inspiredpencil.com
Cation Example Aluminum Oxide Cation For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form. Aluminium oxide contains. Aluminum Oxide Cation.
From sciencenotes.org
Cations and Anions Definitions, Examples, and Differences Aluminum Oxide Cation The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. An aluminum cation is a positively charged aluminum ion that is part of a cationic aluminum. Aluminum Oxide Cation.
From stock.adobe.com
Thin layer of aluminium oxide makes the appearance of aluminium metal Aluminum Oxide Cation Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. The lighter group. Aluminum Oxide Cation.
From study.com
Aluminum Oxide Compound Formula, Properties & Structure Video Aluminum Oxide Cation An aluminum cation is a positively charged aluminum ion that is part of a cationic aluminum complex used as a polymerization catalyst for. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride. Aluminum Oxide Cation.
From www.slideshare.net
Ch 8 ionic compounds Aluminum Oxide Cation Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+,. Aluminum Oxide Cation.
From www.slideserve.com
PPT Chemical Formulas, and Nomenclature ch 9 PowerPoint Presentation Aluminum Oxide Cation An aluminum cation is a positively charged aluminum ion that is part of a cationic aluminum complex used as a polymerization catalyst for. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two. Aluminum Oxide Cation.
From www.youtube.com
Give formation of Aluminium oxide Formation of aluminium oxide by Aluminum Oxide Cation For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or. Aluminum Oxide Cation.
From blog.thepipingmart.com
How to Identify Aluminium Oxide An Overview Aluminum Oxide Cation Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. An aluminum cation is a positively charged aluminum ion that is part of a cationic aluminum complex used as a polymerization catalyst for. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to. Aluminum Oxide Cation.
From www.elementschina.com
Aluminum Oxide Al2O3 CAS No.1344281 Elements China Aluminum Oxide Cation The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. An aluminum cation is a positively charged aluminum ion. Aluminum Oxide Cation.
From chemistry291.blogspot.com
What Is the Chemical Formula for Aluminum Oxide? Aluminum Oxide Cation An aluminum cation is a positively charged aluminum ion that is part of a cationic aluminum complex used as a polymerization catalyst for. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides. Aluminum Oxide Cation.
From www.researchgate.net
Crystal structure of aluminum oxide (Al 2 O 3 ) (a) top view and (b Aluminum Oxide Cation An aluminum cation is a positively charged aluminum ion that is part of a cationic aluminum complex used as a polymerization catalyst for. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride. Aluminum Oxide Cation.
From newatlas.com
Aluminum oxide coatings move like liquids to combat corrosion Aluminum Oxide Cation An aluminum cation is a positively charged aluminum ion that is part of a cationic aluminum complex used as a polymerization catalyst for. Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every. Aluminum Oxide Cation.
From www.slideserve.com
PPT Chemical Formulas, and Nomenclature ch 9 PowerPoint Presentation Aluminum Oxide Cation The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or. Aluminum Oxide Cation.
From mavink.com
Table Of Cations And Anions Aluminum Oxide Cation An aluminum cation is a positively charged aluminum ion that is part of a cationic aluminum complex used as a polymerization catalyst for. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride. Aluminum Oxide Cation.
From www.youtube.com
Chemistry 3Sec The detection of aluminum cation YouTube Aluminum Oxide Cation Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. An aluminum cation. Aluminum Oxide Cation.
From slidetodoc.com
Ionic Bonding Elements are the simplest substances There Aluminum Oxide Cation For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form. An aluminum cation. Aluminum Oxide Cation.
From www.wou.edu
CH150 Chapter 5 Chemical Reactions Chemistry Aluminum Oxide Cation Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. An aluminum cation is a positively charged aluminum ion that is part of a cationic aluminum complex used as a polymerization catalyst for. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to. Aluminum Oxide Cation.
From www.researchgate.net
a. The surface of aluminum oxide. Download Scientific Diagram Aluminum Oxide Cation An aluminum cation is a positively charged aluminum ion that is part of a cationic aluminum complex used as a polymerization catalyst for. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to. Aluminum Oxide Cation.
From www.slideshare.net
Ionic bonding binary Aluminum Oxide Cation Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. An aluminum cation is a positively charged aluminum ion that is part of a cationic aluminum complex used as a polymerization catalyst for. For example, the formula for. Aluminum Oxide Cation.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5980343 Aluminum Oxide Cation For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or. Aluminum Oxide Cation.
From www.youtube.com
Is Al2O3 (Aluminum oxide) Ionic or Covalent? YouTube Aluminum Oxide Cation Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. The lighter group. Aluminum Oxide Cation.
From www.youtube.com
Lewis Dot Structure for Al2O3 (Aluminum Oxide) YouTube Aluminum Oxide Cation The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. For example, the formula for aluminum oxide, al 2. Aluminum Oxide Cation.
From chemistry291.blogspot.com
What Is the Chemical Formula for Aluminum Oxide? Aluminum Oxide Cation Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. An aluminum cation. Aluminum Oxide Cation.
From www.youtube.com
Al 3+ Electron Configuration (Aluminum Ion) YouTube Aluminum Oxide Cation An aluminum cation is a positively charged aluminum ion that is part of a cationic aluminum complex used as a polymerization catalyst for. For example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three. The lighter group 3a metals (aluminum, galium and indium), along with scandium. Aluminum Oxide Cation.