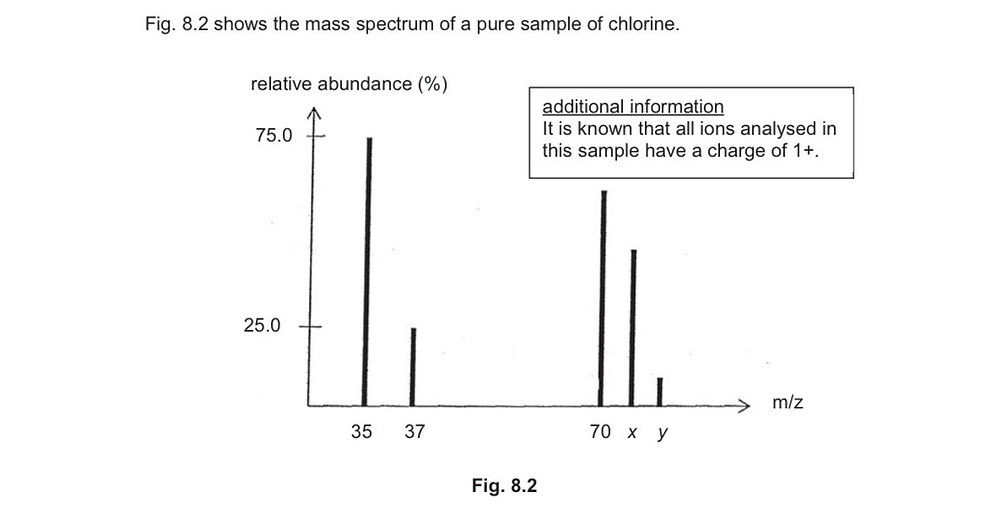
Chlorine And Bromine Mass Spec . Then write the fragmentation processes you might expect, and. Fragments containing both isotopes of br can be seen in the mass spectrum of ethyl bromide: this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. calculate the mass of the molecular ion, and identify the functional groups in the molecule. this is most apparent (at this level) when atoms such as bromine or chlorine are present in a molecule because those elements naturally exist with a. since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a given element. because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have very large and recognizable m+2 peaks. since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a. It also deals briefly with the origin of the m+4 peak in compounds containing two chlorine atoms. this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound.
from www.instantuition.com
It also deals briefly with the origin of the m+4 peak in compounds containing two chlorine atoms. calculate the mass of the molecular ion, and identify the functional groups in the molecule. since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a given element. Fragments containing both isotopes of br can be seen in the mass spectrum of ethyl bromide: Then write the fragmentation processes you might expect, and. since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a. this is most apparent (at this level) when atoms such as bromine or chlorine are present in a molecule because those elements naturally exist with a. this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have very large and recognizable m+2 peaks.
Mass Spectrometry of Chlorine O Level Chemistry
Chlorine And Bromine Mass Spec Fragments containing both isotopes of br can be seen in the mass spectrum of ethyl bromide: this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have very large and recognizable m+2 peaks. this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a given element. since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a. It also deals briefly with the origin of the m+4 peak in compounds containing two chlorine atoms. Fragments containing both isotopes of br can be seen in the mass spectrum of ethyl bromide: calculate the mass of the molecular ion, and identify the functional groups in the molecule. this is most apparent (at this level) when atoms such as bromine or chlorine are present in a molecule because those elements naturally exist with a. Then write the fragmentation processes you might expect, and.
From material-properties.org
Chlorine and Bromine Comparison Properties Material Properties Chlorine And Bromine Mass Spec this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a given element. It also deals briefly with the origin of the m+4 peak in compounds. Chlorine And Bromine Mass Spec.
From www.researchgate.net
Recoveries of bromine (), chlorine (), fluorine (), iodine (), and Chlorine And Bromine Mass Spec It also deals briefly with the origin of the m+4 peak in compounds containing two chlorine atoms. because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have very large and recognizable m+2 peaks. this page explains. Chlorine And Bromine Mass Spec.
From www.slideserve.com
PPT Mass Spectrometry PowerPoint Presentation ID2894903 Chlorine And Bromine Mass Spec since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a. It also deals briefly with the origin of the m+4 peak in compounds containing two chlorine atoms. this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an. Chlorine And Bromine Mass Spec.
From www.researchgate.net
Chlorine and bromine isotope fractionation extents (Δ' h E) of the HOPs Chlorine And Bromine Mass Spec Fragments containing both isotopes of br can be seen in the mass spectrum of ethyl bromide: since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a given element. calculate the mass of the molecular ion, and identify the functional groups in the molecule. this is most apparent (at. Chlorine And Bromine Mass Spec.
From www.slideserve.com
PPT Mass Spectroscopy PowerPoint Presentation, free download ID2407393 Chlorine And Bromine Mass Spec since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a. since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a given element. this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine. Chlorine And Bromine Mass Spec.
From www.instantuition.com
Mass Spectrometry of Chlorine O Level Chemistry Chlorine And Bromine Mass Spec because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have very large and recognizable m+2 peaks. since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a given. Chlorine And Bromine Mass Spec.
From askanydifference.com
Bromine vs Chlorine Difference and Comparison Chlorine And Bromine Mass Spec Fragments containing both isotopes of br can be seen in the mass spectrum of ethyl bromide: since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a. this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic. Chlorine And Bromine Mass Spec.
From www.sliderbase.com
Mass Spectrometry Presentation Chemistry Chlorine And Bromine Mass Spec since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a given element. this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. since a mass spectrometer separates and detects ions of slightly different masses,. Chlorine And Bromine Mass Spec.
From www.numerade.com
SOLVED 15.28 Below are mass spectra for four different chlorine atom Chlorine And Bromine Mass Spec It also deals briefly with the origin of the m+4 peak in compounds containing two chlorine atoms. because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have very large and recognizable m+2 peaks. Then write the fragmentation. Chlorine And Bromine Mass Spec.
From www.youtube.com
How To Easily Draw The Mass Spectra Of Chlorine And Bromine Edexcel Chlorine And Bromine Mass Spec since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a given element. since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a. Fragments containing both isotopes of br can be seen in the mass spectrum of ethyl bromide: Then write. Chlorine And Bromine Mass Spec.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Chlorine And Bromine Mass Spec this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have very large. Chlorine And Bromine Mass Spec.
From chem.libretexts.org
Mass spectrometry 1 Chemistry LibreTexts Chlorine And Bromine Mass Spec this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a given element. calculate the mass of the molecular ion, and identify the functional groups. Chlorine And Bromine Mass Spec.
From www.differencebetween.com
Difference Between Bromine and Chlorine Compare the Difference Chlorine And Bromine Mass Spec since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a given element. since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a. this is most apparent (at this level) when atoms such as bromine or chlorine are present in. Chlorine And Bromine Mass Spec.
From www.chegg.com
Solved Is a chlorine or a bromine present in the molecule Chlorine And Bromine Mass Spec Fragments containing both isotopes of br can be seen in the mass spectrum of ethyl bromide: this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. It also deals briefly with the origin of the m+4 peak in compounds containing two chlorine atoms. because. Chlorine And Bromine Mass Spec.
From www.diffzy.com
Bromine vs. Chlorine What's The Difference In Tabular Form, Points Chlorine And Bromine Mass Spec since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a given element. this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. this page explains how the m+2 peak in a mass spectrum arises. Chlorine And Bromine Mass Spec.
From www.researchgate.net
The mass spectrum of the compound eluting at 24.8 min. The bromine Chlorine And Bromine Mass Spec since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a. It also deals briefly with the origin of the m+4 peak in compounds containing two chlorine atoms. this is most apparent (at this level) when atoms such as bromine or chlorine are present in a molecule because those elements. Chlorine And Bromine Mass Spec.
From www.buzzle.com
Bromine Vs. Chlorine Chlorine And Bromine Mass Spec this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. this is most apparent (at this level) when atoms such as bromine or chlorine are present in a molecule because those elements naturally exist with a. Fragments containing both isotopes of br can be. Chlorine And Bromine Mass Spec.
From www.youtube.com
Exercise 15.24 Using Mass Spectrometry to Determine if Bromine or Chlorine And Bromine Mass Spec Then write the fragmentation processes you might expect, and. since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a given element. this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. since a mass. Chlorine And Bromine Mass Spec.
From www.chegg.com
Solved 6. Point out which of these four mass spectra Chlorine And Bromine Mass Spec since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a given element. this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. this is most apparent (at this level) when atoms such as bromine. Chlorine And Bromine Mass Spec.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Chlorine And Bromine Mass Spec this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have very large. Chlorine And Bromine Mass Spec.
From www.showme.com
Relative abundancies of Chlorine from a mass spectrometer Science Chlorine And Bromine Mass Spec this is most apparent (at this level) when atoms such as bromine or chlorine are present in a molecule because those elements naturally exist with a. Fragments containing both isotopes of br can be seen in the mass spectrum of ethyl bromide: this page explains how the m+2 peak in a mass spectrum arises from the presence of. Chlorine And Bromine Mass Spec.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Chlorine And Bromine Mass Spec since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a given element. since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a. this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine. Chlorine And Bromine Mass Spec.
From blog.sepscience.com
The Role of Isotope Peak Intensities Obtained Using Mass Spectrometry Chlorine And Bromine Mass Spec this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. calculate the mass of the molecular ion, and identify the functional groups in the molecule. Fragments containing both isotopes of br can be seen in the mass spectrum of ethyl bromide: since a. Chlorine And Bromine Mass Spec.
From www.instantuition.com
Mass Spectrometry of Chlorine O Level Chemistry Chlorine And Bromine Mass Spec Then write the fragmentation processes you might expect, and. this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. this is most apparent (at this level) when atoms such as bromine or chlorine are present in a molecule because those elements naturally exist with. Chlorine And Bromine Mass Spec.
From www.researchgate.net
Patterns of the molecular ion with multiple chlorine or bromine atoms Chlorine And Bromine Mass Spec this is most apparent (at this level) when atoms such as bromine or chlorine are present in a molecule because those elements naturally exist with a. calculate the mass of the molecular ion, and identify the functional groups in the molecule. Then write the fragmentation processes you might expect, and. this page explains how the m+2 peak. Chlorine And Bromine Mass Spec.
From blog.chloramineconsulting.com
Chlorine vs. Bromine in Indoor Pools Chlorine And Bromine Mass Spec this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. Then write the fragmentation processes you might expect, and. since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a given element. since a mass. Chlorine And Bromine Mass Spec.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Chlorine And Bromine Mass Spec calculate the mass of the molecular ion, and identify the functional groups in the molecule. because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have very large and recognizable m+2 peaks. It also deals briefly with. Chlorine And Bromine Mass Spec.
From www.slideserve.com
PPT Chapter 12 Mass Spectrometry and Infrared Spectroscopy PowerPoint Chlorine And Bromine Mass Spec Then write the fragmentation processes you might expect, and. because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have very large and recognizable m+2 peaks. It also deals briefly with the origin of the m+4 peak in. Chlorine And Bromine Mass Spec.
From www.slideserve.com
PPT Chem805 Identification of organic and compounds by Chlorine And Bromine Mass Spec Fragments containing both isotopes of br can be seen in the mass spectrum of ethyl bromide: Then write the fragmentation processes you might expect, and. calculate the mass of the molecular ion, and identify the functional groups in the molecule. this is most apparent (at this level) when atoms such as bromine or chlorine are present in a. Chlorine And Bromine Mass Spec.
From www.docbrown.info
C2H4BrCl BrCH2CH2Cl mass spectrum of 1bromo2chloroethane Chlorine And Bromine Mass Spec this is most apparent (at this level) when atoms such as bromine or chlorine are present in a molecule because those elements naturally exist with a. this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. Fragments containing both isotopes of br can be. Chlorine And Bromine Mass Spec.
From mmerevise.co.uk
The Mass Spectrum & Relative Atomic Mass MME Chlorine And Bromine Mass Spec because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have very large and recognizable m+2 peaks. this is most apparent (at this level) when atoms such as bromine or chlorine are present in a molecule because. Chlorine And Bromine Mass Spec.
From www.semanticscholar.org
Figure 1 from Determination of total chlorine and bromine in solid Chlorine And Bromine Mass Spec Then write the fragmentation processes you might expect, and. since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a given element. this is most apparent (at this level) when atoms such as bromine or chlorine are present in a molecule because those elements naturally exist with a. since. Chlorine And Bromine Mass Spec.
From www.slideserve.com
PPT Mass Spectrometry PowerPoint Presentation ID2894903 Chlorine And Bromine Mass Spec Then write the fragmentation processes you might expect, and. since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a given element. because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated. Chlorine And Bromine Mass Spec.
From study.com
How to Identify an Element from Its Mass Spectrum Chemistry Chlorine And Bromine Mass Spec Fragments containing both isotopes of br can be seen in the mass spectrum of ethyl bromide: Then write the fragmentation processes you might expect, and. since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a. because there are two abundant isotopes of both chlorine (about 75% 35 cl and. Chlorine And Bromine Mass Spec.
From www.slideserve.com
PPT Mass Spectrometry PowerPoint Presentation ID2894903 Chlorine And Bromine Mass Spec this is most apparent (at this level) when atoms such as bromine or chlorine are present in a molecule because those elements naturally exist with a. calculate the mass of the molecular ion, and identify the functional groups in the molecule. Then write the fragmentation processes you might expect, and. Fragments containing both isotopes of br can be. Chlorine And Bromine Mass Spec.