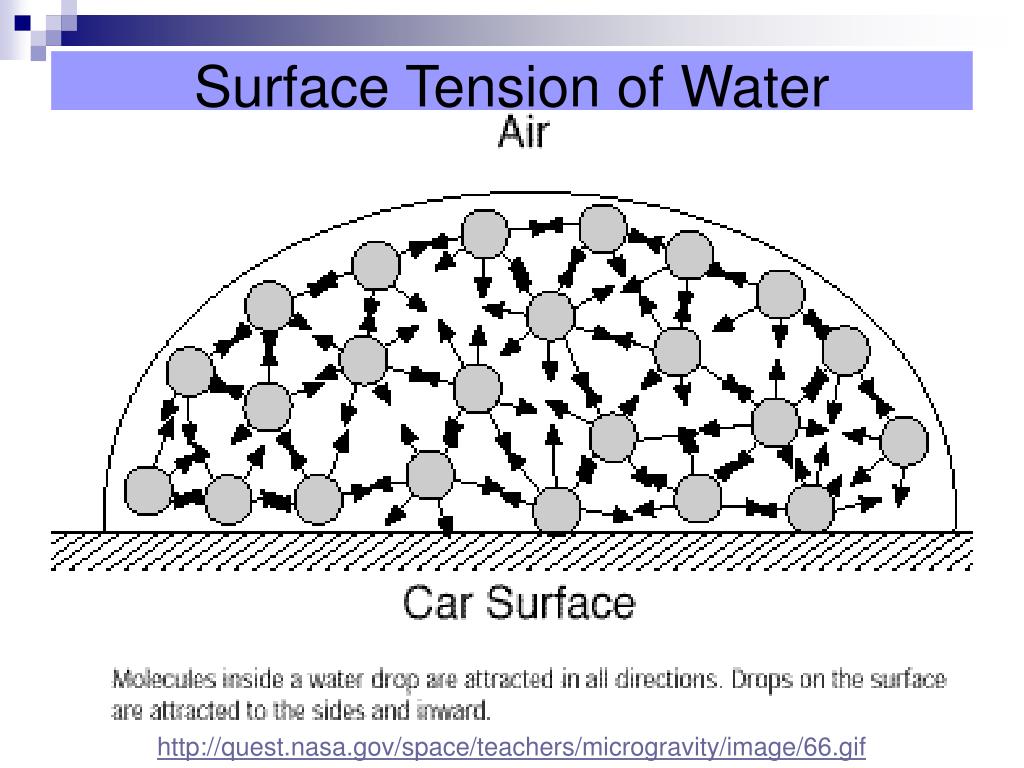
Name Of Water Surface Tension . Since these intermolecular forces vary depending on the nature of the liquid (e.g. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. Surfactants like detergent), each solution exhibits differing surface tension properties. The shape of the molecule and the electronegativity difference between hydrogen and oxygen make water a polar molecule. Water consists of one oxygen atom flanked by two hydrogen atoms. in 1975, working group iii (on special properties) of iaps had critically examined the experimental results of the surface. water has high surface tension, which can be explained by its polarity and hydrogen bonding. Gasoline) or solutes in the liquid (e.g. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). the typical value of the surface tension of water at 20 degrees celsius is about 72 millinewtons per meter (mn/m) or 72 dyn/cm. because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including:. In comparison, organic liquids, such as.
from www.slideserve.com
in 1975, working group iii (on special properties) of iaps had critically examined the experimental results of the surface. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including:. Gasoline) or solutes in the liquid (e.g. Since these intermolecular forces vary depending on the nature of the liquid (e.g. In comparison, organic liquids, such as. the typical value of the surface tension of water at 20 degrees celsius is about 72 millinewtons per meter (mn/m) or 72 dyn/cm. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. Water consists of one oxygen atom flanked by two hydrogen atoms. The shape of the molecule and the electronegativity difference between hydrogen and oxygen make water a polar molecule.
PPT Water and Aqueous Systems PowerPoint Presentation ID565121
Name Of Water Surface Tension the typical value of the surface tension of water at 20 degrees celsius is about 72 millinewtons per meter (mn/m) or 72 dyn/cm. because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including:. Gasoline) or solutes in the liquid (e.g. water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. Since these intermolecular forces vary depending on the nature of the liquid (e.g. in 1975, working group iii (on special properties) of iaps had critically examined the experimental results of the surface. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In comparison, organic liquids, such as. the typical value of the surface tension of water at 20 degrees celsius is about 72 millinewtons per meter (mn/m) or 72 dyn/cm. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water consists of one oxygen atom flanked by two hydrogen atoms. The shape of the molecule and the electronegativity difference between hydrogen and oxygen make water a polar molecule. Surfactants like detergent), each solution exhibits differing surface tension properties.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Name Of Water Surface Tension the typical value of the surface tension of water at 20 degrees celsius is about 72 millinewtons per meter (mn/m) or 72 dyn/cm. because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including:. Water consists of one oxygen atom flanked by two hydrogen atoms. In comparison, organic liquids, such as.. Name Of Water Surface Tension.
From study.com
Surface Tension Definition, Calculation & Examples Video & Lesson Name Of Water Surface Tension Surfactants like detergent), each solution exhibits differing surface tension properties. Water consists of one oxygen atom flanked by two hydrogen atoms. because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including:. in 1975, working group iii (on special properties) of iaps had critically examined the experimental results of the surface.. Name Of Water Surface Tension.
From errantscience.com
ErrantScience Water surface tension Name Of Water Surface Tension In comparison, organic liquids, such as. Since these intermolecular forces vary depending on the nature of the liquid (e.g. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. water has high surface tension, which can be explained by its polarity and. Name Of Water Surface Tension.
From www.yumpu.com
Chapter 9 Properties of Water Properties of Water Surface Tension Name Of Water Surface Tension The shape of the molecule and the electronegativity difference between hydrogen and oxygen make water a polar molecule. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. water has high surface tension, which can be explained by its polarity and hydrogen. Name Of Water Surface Tension.
From www.youtube.com
Surface Tension of Water Explained YouTube Name Of Water Surface Tension water has high surface tension, which can be explained by its polarity and hydrogen bonding. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Since these intermolecular forces vary depending on the nature of the liquid (e.g. The shape of the molecule and the electronegativity difference between hydrogen and oxygen make. Name Of Water Surface Tension.
From www.youtube.com
What is surface tension of water explained in detail? YouTube Name Of Water Surface Tension Since these intermolecular forces vary depending on the nature of the liquid (e.g. because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including:. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. The. Name Of Water Surface Tension.
From errantscience.com
Water surface tension ErrantScience Name Of Water Surface Tension the typical value of the surface tension of water at 20 degrees celsius is about 72 millinewtons per meter (mn/m) or 72 dyn/cm. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. in 1975, working group iii (on special properties). Name Of Water Surface Tension.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 Name Of Water Surface Tension Since these intermolecular forces vary depending on the nature of the liquid (e.g. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. in 1975, working group iii (on special properties) of iaps had critically examined the experimental results of the surface.. Name Of Water Surface Tension.
From www.australianenvironmentaleducation.com.au
Water is found everywhere on earth, so why is it important? Name Of Water Surface Tension the typical value of the surface tension of water at 20 degrees celsius is about 72 millinewtons per meter (mn/m) or 72 dyn/cm. because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including:. Since these intermolecular forces vary depending on the nature of the liquid (e.g. water has a. Name Of Water Surface Tension.
From blog.merocourse.com
What is Surface Tension? Factors Affecting Surface Tension Merocourse Name Of Water Surface Tension Since these intermolecular forces vary depending on the nature of the liquid (e.g. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Gasoline) or solutes in the liquid (e.g. because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including:. surface tension is. Name Of Water Surface Tension.
From www.youtube.com
Water's surface tension physics experiment YouTube Name Of Water Surface Tension water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including:. Water consists of one oxygen atom flanked by two hydrogen atoms. the typical value of the surface tension of water at 20 degrees. Name Of Water Surface Tension.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Name Of Water Surface Tension surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. Water consists of one oxygen atom flanked by two hydrogen atoms. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). because water seems so. Name Of Water Surface Tension.
From www.elephango.com
Tension in the Water Educational Resources K12 Learning, Physical Name Of Water Surface Tension In comparison, organic liquids, such as. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surfactants like detergent), each solution exhibits differing surface tension properties. because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including:. water has high. Name Of Water Surface Tension.
From www.youtube.com
DETERMINING THE SURFACE TENSION OF WATER EASY TO UNDERSTAND YouTube Name Of Water Surface Tension In comparison, organic liquids, such as. the typical value of the surface tension of water at 20 degrees celsius is about 72 millinewtons per meter (mn/m) or 72 dyn/cm. Since these intermolecular forces vary depending on the nature of the liquid (e.g. because water seems so ubiquitous, many people are unaware of the unusual and unique properties of. Name Of Water Surface Tension.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it Name Of Water Surface Tension The shape of the molecule and the electronegativity difference between hydrogen and oxygen make water a polar molecule. Gasoline) or solutes in the liquid (e.g. In comparison, organic liquids, such as. Since these intermolecular forces vary depending on the nature of the liquid (e.g. water has a surface tension of 0.07275 joule per square metre at 20 °c (68. Name Of Water Surface Tension.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID6036532 Name Of Water Surface Tension water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surfactants like detergent), each solution exhibits differing surface tension properties. The shape of the molecule and the electronegativity difference between hydrogen and oxygen make water a polar molecule. in 1975, working group iii (on special properties) of iaps had critically examined the. Name Of Water Surface Tension.
From circuitwiringtray.z13.web.core.windows.net
Surface Tension Of Water Diagram Name Of Water Surface Tension water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Gasoline) or solutes in the liquid (e.g. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. Surfactants like detergent), each solution exhibits differing surface tension. Name Of Water Surface Tension.
From exoorzvyy.blob.core.windows.net
Surface Tension Of Water Lesson Plan at Sabrina Nickerson blog Name Of Water Surface Tension The shape of the molecule and the electronegativity difference between hydrogen and oxygen make water a polar molecule. water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. in 1975, working group. Name Of Water Surface Tension.
From www.slideserve.com
PPT Water and Aqueous Systems PowerPoint Presentation ID565121 Name Of Water Surface Tension Gasoline) or solutes in the liquid (e.g. The shape of the molecule and the electronegativity difference between hydrogen and oxygen make water a polar molecule. because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including:. water has a surface tension of 0.07275 joule per square metre at 20 °c (68. Name Of Water Surface Tension.
From www.slideserve.com
PPT PROPERTIES OF WATER PowerPoint Presentation, free download ID Name Of Water Surface Tension water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). The shape of the molecule and the electronegativity difference between hydrogen and oxygen make water a polar molecule. the typical value of the surface tension of water at 20 degrees celsius is about 72 millinewtons per meter (mn/m) or 72 dyn/cm. . Name Of Water Surface Tension.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces Name Of Water Surface Tension surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the liquid (e.g. because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including:. the typical value of the surface. Name Of Water Surface Tension.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Name Of Water Surface Tension the typical value of the surface tension of water at 20 degrees celsius is about 72 millinewtons per meter (mn/m) or 72 dyn/cm. Surfactants like detergent), each solution exhibits differing surface tension properties. Gasoline) or solutes in the liquid (e.g. The shape of the molecule and the electronegativity difference between hydrogen and oxygen make water a polar molecule. . Name Of Water Surface Tension.
From www.thoughtco.com
Surface Tension Definition in Chemistry Name Of Water Surface Tension water has high surface tension, which can be explained by its polarity and hydrogen bonding. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). The shape of the molecule and the electronegativity difference between hydrogen and oxygen make water a polar molecule. in 1975, working group iii (on special properties). Name Of Water Surface Tension.
From www.slideshare.net
Measuring the Surface Tension of Water by Light Diffraction on Capill… Name Of Water Surface Tension Surfactants like detergent), each solution exhibits differing surface tension properties. In comparison, organic liquids, such as. Gasoline) or solutes in the liquid (e.g. water has high surface tension, which can be explained by its polarity and hydrogen bonding. Since these intermolecular forces vary depending on the nature of the liquid (e.g. in 1975, working group iii (on special. Name Of Water Surface Tension.
From www.biolinscientific.com
Why is surface tension important? Name Of Water Surface Tension Gasoline) or solutes in the liquid (e.g. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including:. water has a surface tension of 0.07275 joule per square metre at. Name Of Water Surface Tension.
From www.expii.com
Surface Tension of Water — Overview & Importance Expii Name Of Water Surface Tension Gasoline) or solutes in the liquid (e.g. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. Surfactants like detergent), each solution exhibits differing surface tension properties. water has a surface tension of 0.07275 joule per square metre at 20 °c (68. Name Of Water Surface Tension.
From www.researchgate.net
2 Schematic representation of surface tension of a hemispherical water Name Of Water Surface Tension Water consists of one oxygen atom flanked by two hydrogen atoms. Surfactants like detergent), each solution exhibits differing surface tension properties. In comparison, organic liquids, such as. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. Gasoline) or solutes in the liquid. Name Of Water Surface Tension.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids Name Of Water Surface Tension Since these intermolecular forces vary depending on the nature of the liquid (e.g. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surfactants like detergent), each solution exhibits differing surface tension properties. because water seems so ubiquitous, many people are unaware of the unusual and unique properties. Name Of Water Surface Tension.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog Name Of Water Surface Tension surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. The shape of the molecule and the electronegativity difference between hydrogen and oxygen make water a polar molecule. Water consists of one oxygen atom flanked by two hydrogen atoms. In comparison, organic liquids,. Name Of Water Surface Tension.
From mavink.com
Water Surface Tension Chart Name Of Water Surface Tension In comparison, organic liquids, such as. Gasoline) or solutes in the liquid (e.g. Water consists of one oxygen atom flanked by two hydrogen atoms. Since these intermolecular forces vary depending on the nature of the liquid (e.g. in 1975, working group iii (on special properties) of iaps had critically examined the experimental results of the surface. Surfactants like detergent),. Name Of Water Surface Tension.
From byjus.com
Explain the surface tension phenomenon with examples. Name Of Water Surface Tension The shape of the molecule and the electronegativity difference between hydrogen and oxygen make water a polar molecule. Since these intermolecular forces vary depending on the nature of the liquid (e.g. In comparison, organic liquids, such as. in 1975, working group iii (on special properties) of iaps had critically examined the experimental results of the surface. water has. Name Of Water Surface Tension.
From www.slideserve.com
PPT The Properties of Water PowerPoint Presentation, free download Name Of Water Surface Tension the typical value of the surface tension of water at 20 degrees celsius is about 72 millinewtons per meter (mn/m) or 72 dyn/cm. Since these intermolecular forces vary depending on the nature of the liquid (e.g. The shape of the molecule and the electronegativity difference between hydrogen and oxygen make water a polar molecule. water has high surface. Name Of Water Surface Tension.
From www.slideserve.com
PPT Water and It’s properties PowerPoint Presentation, free download Name Of Water Surface Tension surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. the typical value of the surface tension of water at 20 degrees celsius is about 72 millinewtons per meter (mn/m) or 72 dyn/cm. In comparison, organic liquids, such as. water has a surface tension of 0.07275 joule. Name Of Water Surface Tension.
From exolnazbp.blob.core.windows.net
Water Surface Tension With Temperature at Amber Holmes blog Name Of Water Surface Tension Surfactants like detergent), each solution exhibits differing surface tension properties. In comparison, organic liquids, such as. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. because water seems so ubiquitous, many people are unaware of the unusual and unique properties of. Name Of Water Surface Tension.
From www.biolinscientific.com
Surface tension of water Why is it so high? Name Of Water Surface Tension In comparison, organic liquids, such as. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. Gasoline) or solutes in the liquid (e.g. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular. Name Of Water Surface Tension.