What Happens To Pressure As Temp Increases . if you continue to pump air into tire (which now has a nearly constant volume), the pressure increases with increasing. if the pressure of the gas is too large (e.g. Hundreds of times larger than atmospheric pressure), or the temperature is too low (e.g. the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal. − 200 c ) there can. Heating a container filled with a mass of gas. scientists noted that for a given amount of a gas (usually expressed in units of moles [n]), if the temperature (t) of the gas was kept. An experiment to investigate the relationship between.
from learningchemistryeasily.blogspot.com
scientists noted that for a given amount of a gas (usually expressed in units of moles [n]), if the temperature (t) of the gas was kept. if the pressure of the gas is too large (e.g. − 200 c ) there can. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal. An experiment to investigate the relationship between. if you continue to pump air into tire (which now has a nearly constant volume), the pressure increases with increasing. Hundreds of times larger than atmospheric pressure), or the temperature is too low (e.g. Heating a container filled with a mass of gas. the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure.
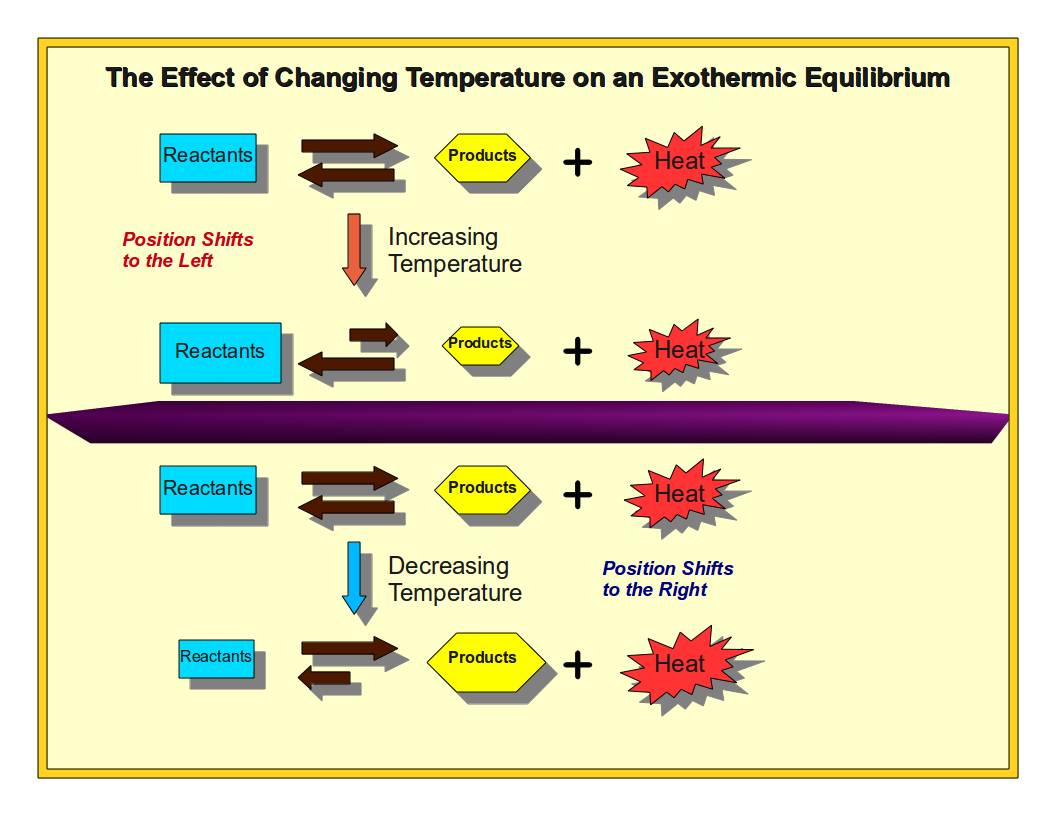
Le Chatelier's Principle Effect of Changing Temperature Learning
What Happens To Pressure As Temp Increases Heating a container filled with a mass of gas. the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure. if you continue to pump air into tire (which now has a nearly constant volume), the pressure increases with increasing. Heating a container filled with a mass of gas. if the pressure of the gas is too large (e.g. scientists noted that for a given amount of a gas (usually expressed in units of moles [n]), if the temperature (t) of the gas was kept. Hundreds of times larger than atmospheric pressure), or the temperature is too low (e.g. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal. An experiment to investigate the relationship between. − 200 c ) there can.
From www.nagwa.com
Question Video Determining How a Change in Temperature Affects the What Happens To Pressure As Temp Increases if you continue to pump air into tire (which now has a nearly constant volume), the pressure increases with increasing. if the pressure of the gas is too large (e.g. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal. the relationships among the volume of. What Happens To Pressure As Temp Increases.
From www.esa.int
ESA Atmospheric temperature changes with altitude What Happens To Pressure As Temp Increases Hundreds of times larger than atmospheric pressure), or the temperature is too low (e.g. scientists noted that for a given amount of a gas (usually expressed in units of moles [n]), if the temperature (t) of the gas was kept. Heating a container filled with a mass of gas. if you continue to pump air into tire (which. What Happens To Pressure As Temp Increases.
From opentextbc.ca
Properties of Liquids Introductory Chemistry 1st Canadian Edition What Happens To Pressure As Temp Increases − 200 c ) there can. if the pressure of the gas is too large (e.g. An experiment to investigate the relationship between. the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure. Heating a container filled with a mass of gas. if you continue to pump air into. What Happens To Pressure As Temp Increases.
From www.slideserve.com
PPT Unit 4 Phases of Matter (Chapters 1314) PowerPoint Presentation What Happens To Pressure As Temp Increases Hundreds of times larger than atmospheric pressure), or the temperature is too low (e.g. An experiment to investigate the relationship between. Heating a container filled with a mass of gas. the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure. if you continue to pump air into tire (which now. What Happens To Pressure As Temp Increases.
From www.slideserve.com
PPT Chemistry 14.1 PowerPoint Presentation, free download ID4874125 What Happens To Pressure As Temp Increases An experiment to investigate the relationship between. − 200 c ) there can. if the pressure of the gas is too large (e.g. Heating a container filled with a mass of gas. Hundreds of times larger than atmospheric pressure), or the temperature is too low (e.g. if you continue to pump air into tire (which now has a. What Happens To Pressure As Temp Increases.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID6271487 What Happens To Pressure As Temp Increases − 200 c ) there can. Hundreds of times larger than atmospheric pressure), or the temperature is too low (e.g. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal. An experiment to investigate the relationship between. the relationships among the volume of a gas and its pressure,. What Happens To Pressure As Temp Increases.
From learningchemistryeasily.blogspot.com
Le Chatelier's Principle Effect of Changing Temperature Learning What Happens To Pressure As Temp Increases scientists noted that for a given amount of a gas (usually expressed in units of moles [n]), if the temperature (t) of the gas was kept. − 200 c ) there can. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal. the relationships among the volume. What Happens To Pressure As Temp Increases.
From kenkidryer.com
Saturation temperature (boiling point) KENKI DRYER What Happens To Pressure As Temp Increases if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal. An experiment to investigate the relationship between. − 200 c ) there can. if you continue to pump air into tire (which now has a nearly constant volume), the pressure increases with increasing. if the pressure of. What Happens To Pressure As Temp Increases.
From ecampusontario.pressbooks.pub
9.5 The Theory Chemistry What Happens To Pressure As Temp Increases Hundreds of times larger than atmospheric pressure), or the temperature is too low (e.g. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal. − 200 c ) there can. scientists noted that for a given amount of a gas (usually expressed in units of moles [n]), if. What Happens To Pressure As Temp Increases.
From www.youtube.com
Constant Pressure Heating of a gas YouTube What Happens To Pressure As Temp Increases the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure. An experiment to investigate the relationship between. scientists noted that for a given amount of a gas (usually expressed in units of moles [n]), if the temperature (t) of the gas was kept. if the pressure of the gas. What Happens To Pressure As Temp Increases.
From saylordotorg.github.io
Effects of Temperature and Pressure on Solubility What Happens To Pressure As Temp Increases the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure. if the pressure of the gas is too large (e.g. if you continue to pump air into tire (which now has a nearly constant volume), the pressure increases with increasing. − 200 c ) there can. Hundreds of times. What Happens To Pressure As Temp Increases.
From socratic.org
How does atmospheric pressure change with altitude? Socratic What Happens To Pressure As Temp Increases scientists noted that for a given amount of a gas (usually expressed in units of moles [n]), if the temperature (t) of the gas was kept. the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure. Hundreds of times larger than atmospheric pressure), or the temperature is too low (e.g.. What Happens To Pressure As Temp Increases.
From general.chemistrysteps.com
Le Chateliers Principle Chemistry Steps What Happens To Pressure As Temp Increases the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure. − 200 c ) there can. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal. scientists noted that for a given amount of a gas (usually expressed in. What Happens To Pressure As Temp Increases.
From www.nagwa.com
Question Video Identifying the Relationship between the Pressure and What Happens To Pressure As Temp Increases Heating a container filled with a mass of gas. if you continue to pump air into tire (which now has a nearly constant volume), the pressure increases with increasing. if the pressure of the gas is too large (e.g. if you had a way to increase pressure with no volume change, then yes, temperature would increase by. What Happens To Pressure As Temp Increases.
From www.atmo.arizona.edu
Changing atmospheric properties with altitude, layers of the atmosphere What Happens To Pressure As Temp Increases Heating a container filled with a mass of gas. Hundreds of times larger than atmospheric pressure), or the temperature is too low (e.g. if you continue to pump air into tire (which now has a nearly constant volume), the pressure increases with increasing. An experiment to investigate the relationship between. the relationships among the volume of a gas. What Happens To Pressure As Temp Increases.
From chem.libretexts.org
6.3 Relationships among Pressure, Temperature, Volume, and Amount What Happens To Pressure As Temp Increases if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal. Heating a container filled with a mass of gas. − 200 c ) there can. if you continue to pump air into tire (which now has a nearly constant volume), the pressure increases with increasing. Hundreds of times. What Happens To Pressure As Temp Increases.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science What Happens To Pressure As Temp Increases Heating a container filled with a mass of gas. − 200 c ) there can. An experiment to investigate the relationship between. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal. if the pressure of the gas is too large (e.g. the relationships among the volume. What Happens To Pressure As Temp Increases.
From www.coursehero.com
[Solved] Use the altitude air pressure calculator (Links to... Course What Happens To Pressure As Temp Increases if the pressure of the gas is too large (e.g. if you continue to pump air into tire (which now has a nearly constant volume), the pressure increases with increasing. − 200 c ) there can. Hundreds of times larger than atmospheric pressure), or the temperature is too low (e.g. if you had a way to increase. What Happens To Pressure As Temp Increases.
From studyrocket.co.uk
Turning Forces and Pressure GCSE Physics AQA Revision Study Rocket What Happens To Pressure As Temp Increases − 200 c ) there can. if the pressure of the gas is too large (e.g. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal. Heating a container filled with a mass of gas. if you continue to pump air into tire (which now has a. What Happens To Pressure As Temp Increases.
From 45.153.231.124
The Effect Of Temperature And Pressure On Solubility Mr C Youtube What Happens To Pressure As Temp Increases Heating a container filled with a mass of gas. the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal. An experiment to investigate the relationship between. scientists noted that. What Happens To Pressure As Temp Increases.
From pressbooks.bccampus.ca
LABORATORY 2 HEAT AND TEMPERATURE IN THE ATMOSPHERE Physical What Happens To Pressure As Temp Increases Heating a container filled with a mass of gas. An experiment to investigate the relationship between. the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure. if you continue to pump air into tire (which now has a nearly constant volume), the pressure increases with increasing. scientists noted that. What Happens To Pressure As Temp Increases.
From www.pinterest.com
phase changes 183 physics Physics, Change, Diagram What Happens To Pressure As Temp Increases An experiment to investigate the relationship between. if the pressure of the gas is too large (e.g. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal. the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure. − 200. What Happens To Pressure As Temp Increases.
From www.grc.nasa.gov
Gas Temperature What Happens To Pressure As Temp Increases if the pressure of the gas is too large (e.g. if you continue to pump air into tire (which now has a nearly constant volume), the pressure increases with increasing. − 200 c ) there can. Heating a container filled with a mass of gas. scientists noted that for a given amount of a gas (usually expressed. What Happens To Pressure As Temp Increases.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent What Happens To Pressure As Temp Increases scientists noted that for a given amount of a gas (usually expressed in units of moles [n]), if the temperature (t) of the gas was kept. if you continue to pump air into tire (which now has a nearly constant volume), the pressure increases with increasing. if you had a way to increase pressure with no volume. What Happens To Pressure As Temp Increases.
From www.youtube.com
Pressure, Volume and Temperature Relationships Chemistry Tutorial What Happens To Pressure As Temp Increases the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure. − 200 c ) there can. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal. An experiment to investigate the relationship between. scientists noted that for a given. What Happens To Pressure As Temp Increases.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download What Happens To Pressure As Temp Increases the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure. Hundreds of times larger than atmospheric pressure), or the temperature is too low (e.g. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal. if the pressure of the. What Happens To Pressure As Temp Increases.
From www.ozmo.io
How Does Water Temperature Affect Chemical Reactions? What Happens To Pressure As Temp Increases if the pressure of the gas is too large (e.g. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal. scientists noted that for a given amount of a gas (usually expressed in units of moles [n]), if the temperature (t) of the gas was kept. An. What Happens To Pressure As Temp Increases.
From www.slideserve.com
PPT 17 Equilibrium (AHL) PowerPoint Presentation, free download ID What Happens To Pressure As Temp Increases Heating a container filled with a mass of gas. the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure. − 200 c ) there can. An experiment to investigate the relationship between. if you continue to pump air into tire (which now has a nearly constant volume), the pressure increases. What Happens To Pressure As Temp Increases.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo What Happens To Pressure As Temp Increases if you continue to pump air into tire (which now has a nearly constant volume), the pressure increases with increasing. the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the. What Happens To Pressure As Temp Increases.
From www.youtube.com
Partial Pressure Change with Temperature (Interactive) YouTube What Happens To Pressure As Temp Increases if you continue to pump air into tire (which now has a nearly constant volume), the pressure increases with increasing. if the pressure of the gas is too large (e.g. Heating a container filled with a mass of gas. if you had a way to increase pressure with no volume change, then yes, temperature would increase by. What Happens To Pressure As Temp Increases.
From studylib.net
Relationship between density, pressure, and temperature What Happens To Pressure As Temp Increases if the pressure of the gas is too large (e.g. Heating a container filled with a mass of gas. the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure. if you continue to pump air into tire (which now has a nearly constant volume), the pressure increases with increasing.. What Happens To Pressure As Temp Increases.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent What Happens To Pressure As Temp Increases Heating a container filled with a mass of gas. if the pressure of the gas is too large (e.g. if you continue to pump air into tire (which now has a nearly constant volume), the pressure increases with increasing. Hundreds of times larger than atmospheric pressure), or the temperature is too low (e.g. − 200 c ) there. What Happens To Pressure As Temp Increases.
From socratic.org
How do mountains affect temperature on both the windward side and the What Happens To Pressure As Temp Increases the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure. scientists noted that for a given amount of a gas (usually expressed in units of moles [n]), if the temperature (t) of the gas was kept. An experiment to investigate the relationship between. if you continue to pump air. What Happens To Pressure As Temp Increases.
From www.theweatherprediction.com
PRESSURE AND TEMPERATURE RELATIONSHIP What Happens To Pressure As Temp Increases if you continue to pump air into tire (which now has a nearly constant volume), the pressure increases with increasing. if you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal. the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in. What Happens To Pressure As Temp Increases.
From www.youtube.com
1.4.6 Solve problems involving temperature, pressure and volume for an What Happens To Pressure As Temp Increases − 200 c ) there can. Hundreds of times larger than atmospheric pressure), or the temperature is too low (e.g. Heating a container filled with a mass of gas. the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure. if you continue to pump air into tire (which now has. What Happens To Pressure As Temp Increases.