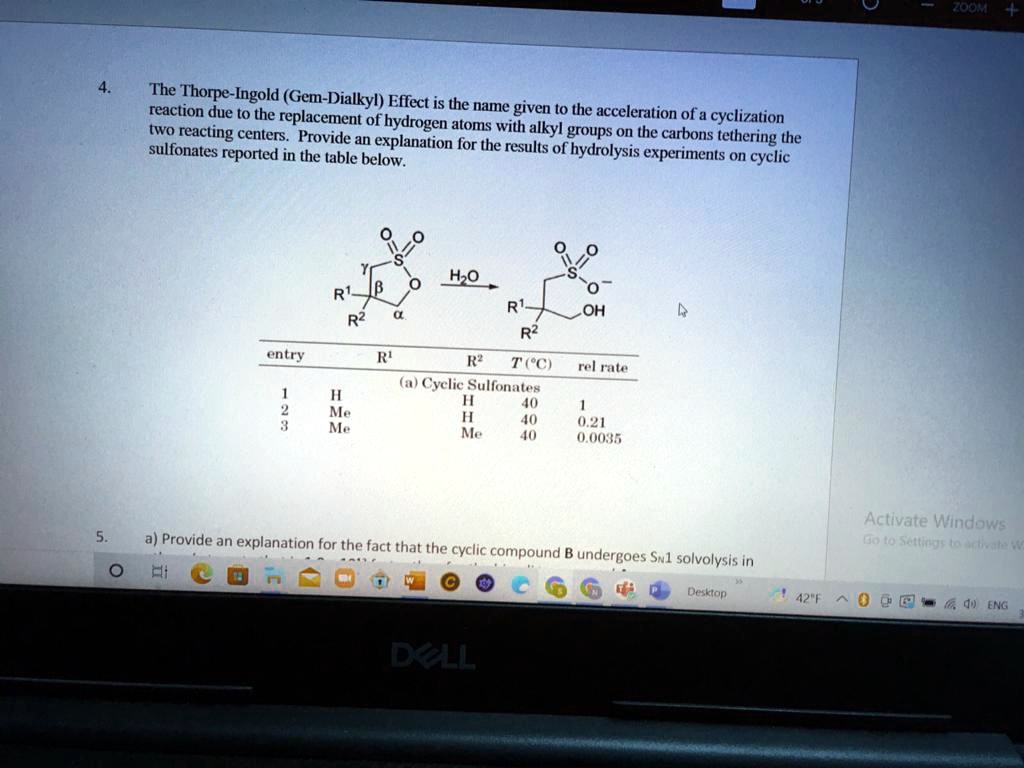
Thorpe Ingold Effect . Dialkyl substitution improved the reaction rates. The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. [4.3.0], stereocenter adjacent to dienophile •. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the other substituents. Find out the general characteristics, criteria, references, examples and experimental tips for this reaction.
from www.numerade.com
The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. Dialkyl substitution improved the reaction rates. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the other substituents. [4.3.0], stereocenter adjacent to dienophile •. Find out the general characteristics, criteria, references, examples and experimental tips for this reaction.
SOLVED The ThorpeIngold (GemDialkyl) Effect is the name given to the
Thorpe Ingold Effect Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the other substituents. The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. Find out the general characteristics, criteria, references, examples and experimental tips for this reaction. Dialkyl substitution improved the reaction rates. [4.3.0], stereocenter adjacent to dienophile •. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the other substituents.
From www.researchgate.net
(PDF) ThorpeIngold Effect and Its Application in Cyclizations in Thorpe Ingold Effect Find out the general characteristics, criteria, references, examples and experimental tips for this reaction. The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the. Thorpe Ingold Effect.
From www.chemistryworld.com
ThorpeIngold effect Opinion Chemistry World Thorpe Ingold Effect Dialkyl substitution improved the reaction rates. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the other substituents. The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. [4.3.0], stereocenter adjacent to dienophile •.. Thorpe Ingold Effect.
From yooannehardacre.blogspot.com
uv vis spectroscopy principle Anne Hardacre Thorpe Ingold Effect Dialkyl substitution improved the reaction rates. Find out the general characteristics, criteria, references, examples and experimental tips for this reaction. The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease. Thorpe Ingold Effect.
From pubs.rsc.org
Diverse strategies for transition metal catalyzed distal C(sp 3 )H Thorpe Ingold Effect Dialkyl substitution improved the reaction rates. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the other substituents. [4.3.0], stereocenter adjacent to dienophile •. The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear.. Thorpe Ingold Effect.
From pubs.acs.org
A Strain Induced Change of Mechanism from a [2 + 2 + 2] to a [2 + 1 + 2 Thorpe Ingold Effect Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the other substituents. The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. Dialkyl substitution improved the reaction rates. [4.3.0], stereocenter adjacent to dienophile •.. Thorpe Ingold Effect.
From pubs.rsc.org
Highly diastereoselective synthesis of tricyclic fusedpyrans by Thorpe Ingold Effect The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the other substituents. Find out the general characteristics, criteria, references, examples and experimental tips for. Thorpe Ingold Effect.
From pubs.rsc.org
Diverse strategies for transition metal catalyzed distal C(sp 3 )H Thorpe Ingold Effect The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. Find out the general characteristics, criteria, references, examples and experimental tips for this reaction. [4.3.0], stereocenter adjacent to dienophile •. Dialkyl substitution improved the reaction rates. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle. Thorpe Ingold Effect.
From pubs.acs.org
Solventfree cyclization of linear dienes using olefin metathesis and Thorpe Ingold Effect The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the other substituents. [4.3.0], stereocenter adjacent to dienophile •. Dialkyl substitution improved the reaction rates.. Thorpe Ingold Effect.
From www.chegg.com
The ThorpeIngold (GemDialkyl) Effect is the name Thorpe Ingold Effect The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. [4.3.0], stereocenter adjacent to dienophile •. Find out the general characteristics, criteria, references, examples and experimental tips for this reaction. Dialkyl substitution improved the reaction rates. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle. Thorpe Ingold Effect.
From www.chemistryworld.com
ThorpeIngold effect Opinion Chemistry World Thorpe Ingold Effect The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. Dialkyl substitution improved the reaction rates. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the other substituents. [4.3.0], stereocenter adjacent to dienophile •.. Thorpe Ingold Effect.
From www.slideserve.com
PPT Organic Chemistry III PowerPoint Presentation, free download ID Thorpe Ingold Effect Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the other substituents. [4.3.0], stereocenter adjacent to dienophile •. Dialkyl substitution improved the reaction rates. The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear.. Thorpe Ingold Effect.
From pubs.rsc.org
A direct route to six and seven membered lactones via γC(sp 3 )H Thorpe Ingold Effect Dialkyl substitution improved the reaction rates. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the other substituents. [4.3.0], stereocenter adjacent to dienophile •. Find out the general characteristics, criteria, references, examples and experimental tips for this reaction. The geminal dialkyl effect is. Thorpe Ingold Effect.
From www.researchgate.net
(PDF) ThorpeIngold effects in cyclizations to fivemembered and six Thorpe Ingold Effect The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. Find out the general characteristics, criteria, references, examples and experimental tips for this reaction. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the. Thorpe Ingold Effect.
From www.coursehero.com
[Solved] 5) (15 points) The ThorpIngold effect (also called the Thorpe Ingold Effect Dialkyl substitution improved the reaction rates. Find out the general characteristics, criteria, references, examples and experimental tips for this reaction. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the other substituents. The geminal dialkyl effect is widely used in organic synthesis to. Thorpe Ingold Effect.
From pubs.rsc.org
sp 3 CH activation via exo type directing groups Chemical Science Thorpe Ingold Effect [4.3.0], stereocenter adjacent to dienophile •. The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the other substituents. Dialkyl substitution improved the reaction rates.. Thorpe Ingold Effect.
From onlinelibrary.wiley.com
Enantioselective Photochemical Organocascade Catalysis Woźniak 2018 Thorpe Ingold Effect Dialkyl substitution improved the reaction rates. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the other substituents. The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. Find out the general characteristics, criteria,. Thorpe Ingold Effect.
From www.semanticscholar.org
Table 1 from Accelerating influence of the gemdifluoromethylene group Thorpe Ingold Effect Find out the general characteristics, criteria, references, examples and experimental tips for this reaction. Dialkyl substitution improved the reaction rates. [4.3.0], stereocenter adjacent to dienophile •. The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle. Thorpe Ingold Effect.
From www.academia.edu
(PDF) ThorpeIngold effect in the reaction of vicinal amino primary Thorpe Ingold Effect [4.3.0], stereocenter adjacent to dienophile •. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the other substituents. Dialkyl substitution improved the reaction rates. The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear.. Thorpe Ingold Effect.
From www.chemistryworld.com
ThorpeIngold effect Opinion Chemistry World Thorpe Ingold Effect Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the other substituents. The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. [4.3.0], stereocenter adjacent to dienophile •. Find out the general characteristics, criteria,. Thorpe Ingold Effect.
From www.chemistryworld.com
ThorpeIngold effect Opinion Chemistry World Thorpe Ingold Effect The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. Dialkyl substitution improved the reaction rates. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the other substituents. [4.3.0], stereocenter adjacent to dienophile •.. Thorpe Ingold Effect.
From www.cornellab.com
publications — CORNELLAB Thorpe Ingold Effect The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. [4.3.0], stereocenter adjacent to dienophile •. Find out the general characteristics, criteria, references, examples and experimental tips for this reaction. Dialkyl substitution improved the reaction rates. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle. Thorpe Ingold Effect.
From www.semanticscholar.org
Star polymer synthesis viaλorthogonal photochemistry. Semantic Scholar Thorpe Ingold Effect The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. Find out the general characteristics, criteria, references, examples and experimental tips for this reaction. [4.3.0], stereocenter adjacent to dienophile •. Dialkyl substitution improved the reaction rates. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle. Thorpe Ingold Effect.
From www.chemistryworld.com
ThorpeIngold effect Opinion Chemistry World Thorpe Ingold Effect Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the other substituents. Dialkyl substitution improved the reaction rates. The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. Find out the general characteristics, criteria,. Thorpe Ingold Effect.
From www.numerade.com
SOLVED The ThorpeIngold (GemDialkyl) Effect is the name given to the Thorpe Ingold Effect The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. Find out the general characteristics, criteria, references, examples and experimental tips for this reaction. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the. Thorpe Ingold Effect.
From www.researchgate.net
(PDF) ThorpeIngold Effect and Its Application in Cyclizations in Thorpe Ingold Effect [4.3.0], stereocenter adjacent to dienophile •. Find out the general characteristics, criteria, references, examples and experimental tips for this reaction. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the other substituents. The geminal dialkyl effect is widely used in organic synthesis to. Thorpe Ingold Effect.
From pubs.acs.org
Thorpe−Ingold Effect on Photoinduced Electron Transfer of Thorpe Ingold Effect Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the other substituents. The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. Dialkyl substitution improved the reaction rates. Find out the general characteristics, criteria,. Thorpe Ingold Effect.
From www.youtube.com
ThorpeIngold effect explanation with examples for gate Thorpe Ingold Effect Find out the general characteristics, criteria, references, examples and experimental tips for this reaction. The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. Dialkyl substitution improved the reaction rates. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease. Thorpe Ingold Effect.
From www.researchgate.net
ThorpeIngold effect Download Scientific Diagram Thorpe Ingold Effect The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. Find out the general characteristics, criteria, references, examples and experimental tips for this reaction. Dialkyl substitution improved the reaction rates. [4.3.0], stereocenter adjacent to dienophile •. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle. Thorpe Ingold Effect.
From www.coursehero.com
[Solved] 5) (15 points) The ThorpIngold effect (also called the Thorpe Ingold Effect Dialkyl substitution improved the reaction rates. Find out the general characteristics, criteria, references, examples and experimental tips for this reaction. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the other substituents. The geminal dialkyl effect is widely used in organic synthesis to. Thorpe Ingold Effect.
From www.chemistryworld.com
Curtius rearrangement Opinion Chemistry World Thorpe Ingold Effect The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. [4.3.0], stereocenter adjacent to dienophile •. Find out the general characteristics, criteria, references, examples and experimental tips for this reaction. Dialkyl substitution improved the reaction rates. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle. Thorpe Ingold Effect.
From pubs.acs.org
Harnessing ThorpeIngold Dialkylation to Access HighHillPercentage pH Thorpe Ingold Effect [4.3.0], stereocenter adjacent to dienophile •. Find out the general characteristics, criteria, references, examples and experimental tips for this reaction. The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease. Thorpe Ingold Effect.
From www.researchgate.net
(PDF) ThorpeIngold Effect and Its Application in Cyclizations in Thorpe Ingold Effect [4.3.0], stereocenter adjacent to dienophile •. Dialkyl substitution improved the reaction rates. The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. Find out the general characteristics, criteria, references, examples and experimental tips for this reaction. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle. Thorpe Ingold Effect.
From faculti.net
ThorpeIngold Effect on Polymer Conformations Faculti Thorpe Ingold Effect Find out the general characteristics, criteria, references, examples and experimental tips for this reaction. [4.3.0], stereocenter adjacent to dienophile •. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the other substituents. Dialkyl substitution improved the reaction rates. The geminal dialkyl effect is. Thorpe Ingold Effect.
From www.researchgate.net
(PDF) The ThorpeIngold effect promotes tandem 6exo dig cyclization Thorpe Ingold Effect Find out the general characteristics, criteria, references, examples and experimental tips for this reaction. Dialkyl substitution improved the reaction rates. The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease. Thorpe Ingold Effect.
From pubs.rsc.org
Macrocyclisation of small peptides enabled by oxetane incorporation Thorpe Ingold Effect Dialkyl substitution improved the reaction rates. The geminal dialkyl effect is widely used in organic synthesis to promote cyclization reactions, but its origin remains unclear. Thorpe and ingold suggested that repulsion between dialkyl groups causes an increase in the angle x between them and a decrease in the angle y between the other substituents. [4.3.0], stereocenter adjacent to dienophile •.. Thorpe Ingold Effect.