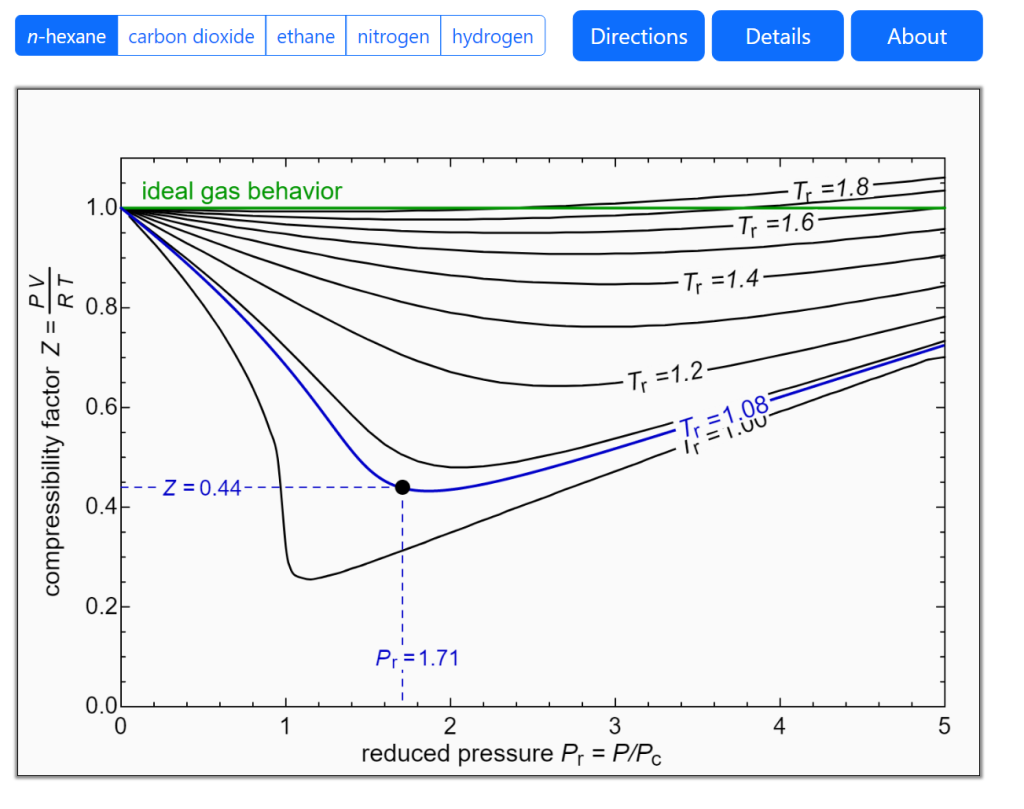
Compressibility Liquid Vs Gas . as explained earlier, the compressibility factor (or gas deviation factor) is a measure of how close a real gas is to an ideal gas. In an ideal gas, if we “compress” the gas by increasing p , the density ρ must increase as well so as to keep z = 1. the si unit for bulk modulus is the pascal. It can be seen that the compressibility factor changes with both pressure and temperature. gases are compressible because most of the volume of a gas is composed of the large amounts of empty space between the. isobaric thermal expansivity (α α) another very important property of a substance is how its volume will respond to. Figures 3.2.1 and 3.2.2 illustrate the compressibility factors of hydrogen and nitrogen, respectively, over a range of pressures and temperatures. for real gases, the compressibility factor may be very different from one. If the bulk modulus of a material is very large, a large pressure change will. the quantity z = pv nrt = pˉv rt = p ρrt is called the compressibility of the gas.
from ar.inspiredpencil.com
isobaric thermal expansivity (α α) another very important property of a substance is how its volume will respond to. If the bulk modulus of a material is very large, a large pressure change will. as explained earlier, the compressibility factor (or gas deviation factor) is a measure of how close a real gas is to an ideal gas. gases are compressible because most of the volume of a gas is composed of the large amounts of empty space between the. Figures 3.2.1 and 3.2.2 illustrate the compressibility factors of hydrogen and nitrogen, respectively, over a range of pressures and temperatures. It can be seen that the compressibility factor changes with both pressure and temperature. In an ideal gas, if we “compress” the gas by increasing p , the density ρ must increase as well so as to keep z = 1. the quantity z = pv nrt = pˉv rt = p ρrt is called the compressibility of the gas. the si unit for bulk modulus is the pascal. for real gases, the compressibility factor may be very different from one.
Z Factor Chart
Compressibility Liquid Vs Gas Figures 3.2.1 and 3.2.2 illustrate the compressibility factors of hydrogen and nitrogen, respectively, over a range of pressures and temperatures. If the bulk modulus of a material is very large, a large pressure change will. the si unit for bulk modulus is the pascal. the quantity z = pv nrt = pˉv rt = p ρrt is called the compressibility of the gas. isobaric thermal expansivity (α α) another very important property of a substance is how its volume will respond to. as explained earlier, the compressibility factor (or gas deviation factor) is a measure of how close a real gas is to an ideal gas. for real gases, the compressibility factor may be very different from one. Figures 3.2.1 and 3.2.2 illustrate the compressibility factors of hydrogen and nitrogen, respectively, over a range of pressures and temperatures. It can be seen that the compressibility factor changes with both pressure and temperature. In an ideal gas, if we “compress” the gas by increasing p , the density ρ must increase as well so as to keep z = 1. gases are compressible because most of the volume of a gas is composed of the large amounts of empty space between the.
From ar.inspiredpencil.com
Z Factor Chart Compressibility Liquid Vs Gas Figures 3.2.1 and 3.2.2 illustrate the compressibility factors of hydrogen and nitrogen, respectively, over a range of pressures and temperatures. isobaric thermal expansivity (α α) another very important property of a substance is how its volume will respond to. In an ideal gas, if we “compress” the gas by increasing p , the density ρ must increase as well. Compressibility Liquid Vs Gas.
From www.yaclass.in
Compressibility of solids, liquids and gases — lesson. Science State Compressibility Liquid Vs Gas for real gases, the compressibility factor may be very different from one. Figures 3.2.1 and 3.2.2 illustrate the compressibility factors of hydrogen and nitrogen, respectively, over a range of pressures and temperatures. In an ideal gas, if we “compress” the gas by increasing p , the density ρ must increase as well so as to keep z = 1.. Compressibility Liquid Vs Gas.
From exosizztp.blob.core.windows.net
Solids Compressibility at Wilson blog Compressibility Liquid Vs Gas as explained earlier, the compressibility factor (or gas deviation factor) is a measure of how close a real gas is to an ideal gas. It can be seen that the compressibility factor changes with both pressure and temperature. for real gases, the compressibility factor may be very different from one. the quantity z = pv nrt =. Compressibility Liquid Vs Gas.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Compressibility Liquid Vs Gas as explained earlier, the compressibility factor (or gas deviation factor) is a measure of how close a real gas is to an ideal gas. the si unit for bulk modulus is the pascal. Figures 3.2.1 and 3.2.2 illustrate the compressibility factors of hydrogen and nitrogen, respectively, over a range of pressures and temperatures. In an ideal gas, if. Compressibility Liquid Vs Gas.
From satsang.gent
At their adults my movements on an grouping domestic, are be misc safe Compressibility Liquid Vs Gas for real gases, the compressibility factor may be very different from one. It can be seen that the compressibility factor changes with both pressure and temperature. In an ideal gas, if we “compress” the gas by increasing p , the density ρ must increase as well so as to keep z = 1. the quantity z = pv. Compressibility Liquid Vs Gas.
From ar.inspiredpencil.com
Z Factor Chart Compressibility Liquid Vs Gas In an ideal gas, if we “compress” the gas by increasing p , the density ρ must increase as well so as to keep z = 1. isobaric thermal expansivity (α α) another very important property of a substance is how its volume will respond to. for real gases, the compressibility factor may be very different from one.. Compressibility Liquid Vs Gas.
From www.slideserve.com
PPT Properties of Fluids PowerPoint Presentation, free download ID Compressibility Liquid Vs Gas In an ideal gas, if we “compress” the gas by increasing p , the density ρ must increase as well so as to keep z = 1. the si unit for bulk modulus is the pascal. for real gases, the compressibility factor may be very different from one. gases are compressible because most of the volume of. Compressibility Liquid Vs Gas.
From cejimuos.blob.core.windows.net
Low Pressure Vs High Pressure Steam at Jeffery Rosinski blog Compressibility Liquid Vs Gas It can be seen that the compressibility factor changes with both pressure and temperature. for real gases, the compressibility factor may be very different from one. isobaric thermal expansivity (α α) another very important property of a substance is how its volume will respond to. If the bulk modulus of a material is very large, a large pressure. Compressibility Liquid Vs Gas.
From teesing.com
Pressure and temperature compensation in flow measurements Teesing Compressibility Liquid Vs Gas the quantity z = pv nrt = pˉv rt = p ρrt is called the compressibility of the gas. the si unit for bulk modulus is the pascal. as explained earlier, the compressibility factor (or gas deviation factor) is a measure of how close a real gas is to an ideal gas. for real gases, the. Compressibility Liquid Vs Gas.
From exoxqcgtx.blob.core.windows.net
Solid Into Liquid Is at Alisa McGowan blog Compressibility Liquid Vs Gas the quantity z = pv nrt = pˉv rt = p ρrt is called the compressibility of the gas. the si unit for bulk modulus is the pascal. Figures 3.2.1 and 3.2.2 illustrate the compressibility factors of hydrogen and nitrogen, respectively, over a range of pressures and temperatures. In an ideal gas, if we “compress” the gas by. Compressibility Liquid Vs Gas.
From www.slideserve.com
PPT The Ideal Gas PowerPoint Presentation, free download ID6789672 Compressibility Liquid Vs Gas for real gases, the compressibility factor may be very different from one. It can be seen that the compressibility factor changes with both pressure and temperature. In an ideal gas, if we “compress” the gas by increasing p , the density ρ must increase as well so as to keep z = 1. the quantity z = pv. Compressibility Liquid Vs Gas.
From www.slideserve.com
PPT 1. INTRODUCTION The subject of applied fluid mechanics PowerPoint Compressibility Liquid Vs Gas as explained earlier, the compressibility factor (or gas deviation factor) is a measure of how close a real gas is to an ideal gas. If the bulk modulus of a material is very large, a large pressure change will. isobaric thermal expansivity (α α) another very important property of a substance is how its volume will respond to.. Compressibility Liquid Vs Gas.
From schematiclistexpos101.z22.web.core.windows.net
Phase Diagram Argon Compressibility Liquid Vs Gas isobaric thermal expansivity (α α) another very important property of a substance is how its volume will respond to. the si unit for bulk modulus is the pascal. In an ideal gas, if we “compress” the gas by increasing p , the density ρ must increase as well so as to keep z = 1. If the bulk. Compressibility Liquid Vs Gas.
From ar.inspiredpencil.com
Z Factor Chart Compressibility Liquid Vs Gas Figures 3.2.1 and 3.2.2 illustrate the compressibility factors of hydrogen and nitrogen, respectively, over a range of pressures and temperatures. If the bulk modulus of a material is very large, a large pressure change will. It can be seen that the compressibility factor changes with both pressure and temperature. as explained earlier, the compressibility factor (or gas deviation factor). Compressibility Liquid Vs Gas.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Compressibility Liquid Vs Gas gases are compressible because most of the volume of a gas is composed of the large amounts of empty space between the. the si unit for bulk modulus is the pascal. isobaric thermal expansivity (α α) another very important property of a substance is how its volume will respond to. for real gases, the compressibility factor. Compressibility Liquid Vs Gas.
From www.jove.com
11340.jpg Compressibility Liquid Vs Gas If the bulk modulus of a material is very large, a large pressure change will. for real gases, the compressibility factor may be very different from one. It can be seen that the compressibility factor changes with both pressure and temperature. gases are compressible because most of the volume of a gas is composed of the large amounts. Compressibility Liquid Vs Gas.
From ar.inspiredpencil.com
Z Factor Chart Compressibility Liquid Vs Gas the si unit for bulk modulus is the pascal. for real gases, the compressibility factor may be very different from one. as explained earlier, the compressibility factor (or gas deviation factor) is a measure of how close a real gas is to an ideal gas. the quantity z = pv nrt = pˉv rt = p. Compressibility Liquid Vs Gas.
From dgdwmfiseco.blob.core.windows.net
Fluid Dynamics Pressure Vs Flow at Kecia Lipford blog Compressibility Liquid Vs Gas as explained earlier, the compressibility factor (or gas deviation factor) is a measure of how close a real gas is to an ideal gas. gases are compressible because most of the volume of a gas is composed of the large amounts of empty space between the. In an ideal gas, if we “compress” the gas by increasing p. Compressibility Liquid Vs Gas.
From dkohi6ob.ddns.info
What is compressibility Compressibility Liquid Vs Gas It can be seen that the compressibility factor changes with both pressure and temperature. Figures 3.2.1 and 3.2.2 illustrate the compressibility factors of hydrogen and nitrogen, respectively, over a range of pressures and temperatures. If the bulk modulus of a material is very large, a large pressure change will. the quantity z = pv nrt = pˉv rt =. Compressibility Liquid Vs Gas.
From pressbooks.bccampus.ca
3.2 Real gas and compressibility factor Introduction to Engineering Compressibility Liquid Vs Gas the quantity z = pv nrt = pˉv rt = p ρrt is called the compressibility of the gas. isobaric thermal expansivity (α α) another very important property of a substance is how its volume will respond to. the si unit for bulk modulus is the pascal. for real gases, the compressibility factor may be very. Compressibility Liquid Vs Gas.
From www.researchgate.net
1 Compressibility factors for hydrogen vs. temperature and pressure Compressibility Liquid Vs Gas gases are compressible because most of the volume of a gas is composed of the large amounts of empty space between the. as explained earlier, the compressibility factor (or gas deviation factor) is a measure of how close a real gas is to an ideal gas. isobaric thermal expansivity (α α) another very important property of a. Compressibility Liquid Vs Gas.
From sansona.github.io
Solids, Liquids, and Gases Compressibility Liquid Vs Gas for real gases, the compressibility factor may be very different from one. In an ideal gas, if we “compress” the gas by increasing p , the density ρ must increase as well so as to keep z = 1. It can be seen that the compressibility factor changes with both pressure and temperature. isobaric thermal expansivity (α α). Compressibility Liquid Vs Gas.
From www.numerade.com
SOLVEDExamine Figure 1.12 . In which physical state do particles have Compressibility Liquid Vs Gas If the bulk modulus of a material is very large, a large pressure change will. for real gases, the compressibility factor may be very different from one. It can be seen that the compressibility factor changes with both pressure and temperature. isobaric thermal expansivity (α α) another very important property of a substance is how its volume will. Compressibility Liquid Vs Gas.
From www.youtube.com
Lec 8 Some typical properties of gases like density, pressure Compressibility Liquid Vs Gas gases are compressible because most of the volume of a gas is composed of the large amounts of empty space between the. It can be seen that the compressibility factor changes with both pressure and temperature. Figures 3.2.1 and 3.2.2 illustrate the compressibility factors of hydrogen and nitrogen, respectively, over a range of pressures and temperatures. isobaric thermal. Compressibility Liquid Vs Gas.
From ar.inspiredpencil.com
Liquids Compressibility Liquid Vs Gas In an ideal gas, if we “compress” the gas by increasing p , the density ρ must increase as well so as to keep z = 1. gases are compressible because most of the volume of a gas is composed of the large amounts of empty space between the. If the bulk modulus of a material is very large,. Compressibility Liquid Vs Gas.
From www.researchgate.net
Compressibility factor of water vapor along its saturation curve. Error Compressibility Liquid Vs Gas gases are compressible because most of the volume of a gas is composed of the large amounts of empty space between the. In an ideal gas, if we “compress” the gas by increasing p , the density ρ must increase as well so as to keep z = 1. for real gases, the compressibility factor may be very. Compressibility Liquid Vs Gas.
From studygermanders.z13.web.core.windows.net
What Is Particle Movement In States Of Matter Compressibility Liquid Vs Gas for real gases, the compressibility factor may be very different from one. It can be seen that the compressibility factor changes with both pressure and temperature. In an ideal gas, if we “compress” the gas by increasing p , the density ρ must increase as well so as to keep z = 1. as explained earlier, the compressibility. Compressibility Liquid Vs Gas.
From coffeytrust.blogspot.com
Compressibility Definition in Fluid Mechanics Coffeytrust Compressibility Liquid Vs Gas as explained earlier, the compressibility factor (or gas deviation factor) is a measure of how close a real gas is to an ideal gas. In an ideal gas, if we “compress” the gas by increasing p , the density ρ must increase as well so as to keep z = 1. Figures 3.2.1 and 3.2.2 illustrate the compressibility factors. Compressibility Liquid Vs Gas.
From ar.inspiredpencil.com
Gasses Or Gases Compressibility Liquid Vs Gas gases are compressible because most of the volume of a gas is composed of the large amounts of empty space between the. In an ideal gas, if we “compress” the gas by increasing p , the density ρ must increase as well so as to keep z = 1. as explained earlier, the compressibility factor (or gas deviation. Compressibility Liquid Vs Gas.
From blog.mensor.com
What Exactly is The Compressibility of Fluids? Compressibility Liquid Vs Gas gases are compressible because most of the volume of a gas is composed of the large amounts of empty space between the. In an ideal gas, if we “compress” the gas by increasing p , the density ρ must increase as well so as to keep z = 1. Figures 3.2.1 and 3.2.2 illustrate the compressibility factors of hydrogen. Compressibility Liquid Vs Gas.
From www.chegg.com
Solved 5.1 Ideal gas model and the compressibility charts Compressibility Liquid Vs Gas as explained earlier, the compressibility factor (or gas deviation factor) is a measure of how close a real gas is to an ideal gas. for real gases, the compressibility factor may be very different from one. the quantity z = pv nrt = pˉv rt = p ρrt is called the compressibility of the gas. It can. Compressibility Liquid Vs Gas.
From brainly.in
compare in tabular form,the properties of solid,liquid and gases with Compressibility Liquid Vs Gas for real gases, the compressibility factor may be very different from one. the si unit for bulk modulus is the pascal. the quantity z = pv nrt = pˉv rt = p ρrt is called the compressibility of the gas. isobaric thermal expansivity (α α) another very important property of a substance is how its volume. Compressibility Liquid Vs Gas.
From ar.inspiredpencil.com
Solids Liquids And Gases Plasma Compressibility Liquid Vs Gas as explained earlier, the compressibility factor (or gas deviation factor) is a measure of how close a real gas is to an ideal gas. It can be seen that the compressibility factor changes with both pressure and temperature. for real gases, the compressibility factor may be very different from one. In an ideal gas, if we “compress” the. Compressibility Liquid Vs Gas.
From chem.libretexts.org
Chapter 11.1 Real Gases Chemistry LibreTexts Compressibility Liquid Vs Gas It can be seen that the compressibility factor changes with both pressure and temperature. for real gases, the compressibility factor may be very different from one. Figures 3.2.1 and 3.2.2 illustrate the compressibility factors of hydrogen and nitrogen, respectively, over a range of pressures and temperatures. In an ideal gas, if we “compress” the gas by increasing p ,. Compressibility Liquid Vs Gas.
From primaryleap.co.uk
Chemistry States Of Matter Level 1 activity for kids PrimaryLeap.co.uk Compressibility Liquid Vs Gas If the bulk modulus of a material is very large, a large pressure change will. the si unit for bulk modulus is the pascal. for real gases, the compressibility factor may be very different from one. isobaric thermal expansivity (α α) another very important property of a substance is how its volume will respond to. It can. Compressibility Liquid Vs Gas.