Titration Reaction Sodium Hydroxide . the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Includes kit list and safety instructions. a titration is an analytical procedure used to determine the concentration of a sample by reacting it with a standard solution. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. (3) the titration setup a titration experiment is one where one attempts to determine the concentration of a sample solution by. The concept and technique of titration.
from www.chegg.com
a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. The concept and technique of titration. (3) the titration setup a titration experiment is one where one attempts to determine the concentration of a sample solution by. Includes kit list and safety instructions. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. a titration is an analytical procedure used to determine the concentration of a sample by reacting it with a standard solution.
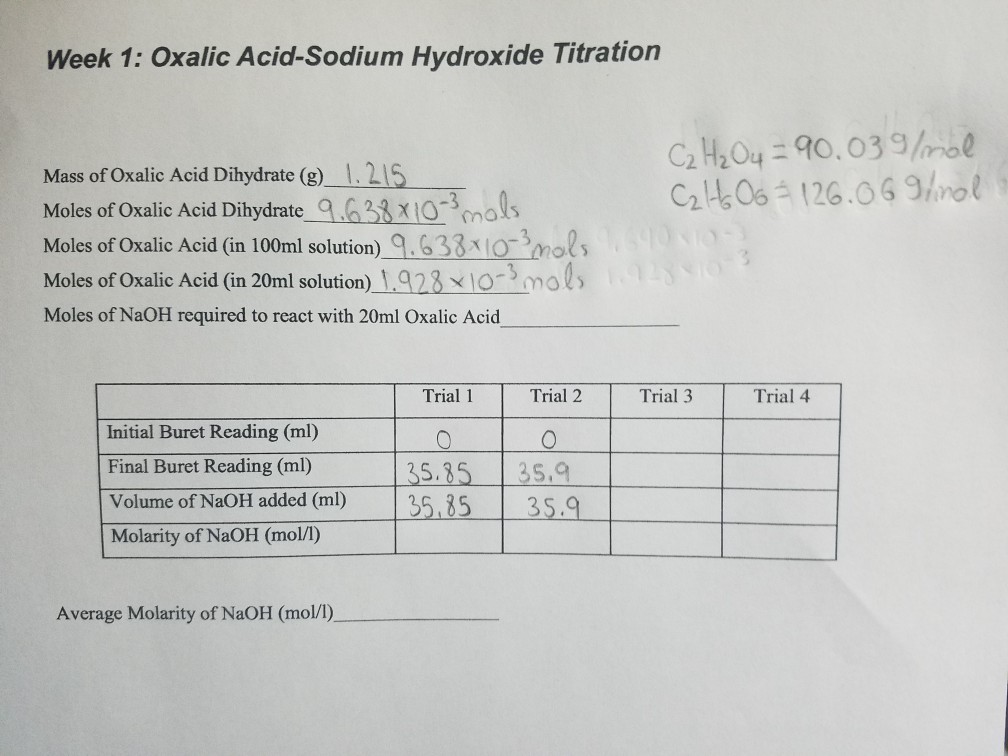
Solved Week 1 Oxalic AcidSodium Hydroxide Titration Mass
Titration Reaction Sodium Hydroxide a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. a titration is an analytical procedure used to determine the concentration of a sample by reacting it with a standard solution. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The concept and technique of titration. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. (3) the titration setup a titration experiment is one where one attempts to determine the concentration of a sample solution by. Includes kit list and safety instructions.
From studylib.net
Standardizing a Sodium Hydroxide (NaOH) Solution Titration Reaction Sodium Hydroxide a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. a titration is an analytical procedure used to determine the concentration of a sample by reacting it with a standard solution. The concept and technique of titration. use this class practical to. Titration Reaction Sodium Hydroxide.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Titration Reaction Sodium Hydroxide a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Includes kit list and safety instructions. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. (3) the titration setup a titration experiment is. Titration Reaction Sodium Hydroxide.
From cekwurnf.blob.core.windows.net
Titration Ratio at Carol Byrd blog Titration Reaction Sodium Hydroxide The concept and technique of titration. Includes kit list and safety instructions. a titration is an analytical procedure used to determine the concentration of a sample by reacting it with a standard solution. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to.. Titration Reaction Sodium Hydroxide.
From www.chegg.com
Solved Use the following titration curve for the first four Titration Reaction Sodium Hydroxide Includes kit list and safety instructions. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. (3) the titration setup a titration experiment is one where one attempts to determine the concentration of a sample solution by. a titration is an analytical. Titration Reaction Sodium Hydroxide.
From www.chegg.com
Solved Week 1 Oxalic AcidSodium Hydroxide Titration Mass Titration Reaction Sodium Hydroxide Includes kit list and safety instructions. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. a titration is an analytical procedure used to determine the concentration of a sample by reacting it with a standard solution. a titration is an experiment where a volume of a solution of. Titration Reaction Sodium Hydroxide.
From mavink.com
Sodium Hydroxide Viscosity Chart Titration Reaction Sodium Hydroxide Includes kit list and safety instructions. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. (3) the titration setup a titration experiment is. Titration Reaction Sodium Hydroxide.
From www.chegg.com
Solved Method Titration of sodium hydroxide and hydrochloric Titration Reaction Sodium Hydroxide Includes kit list and safety instructions. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. (3) the titration setup a titration experiment is one where one attempts to determine the concentration of a sample solution by. a titration is an analytical procedure used to determine the. Titration Reaction Sodium Hydroxide.
From fr.slideserve.com
PPT Common Types of Reactions PowerPoint Presentation, free download Titration Reaction Sodium Hydroxide Includes kit list and safety instructions. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The concept and technique of titration. a titration is an experiment where a. Titration Reaction Sodium Hydroxide.
From www.numerade.com
SOLVED The following data were collected during a titration Calculate Titration Reaction Sodium Hydroxide a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. a titration is an analytical procedure used to determine the concentration of. Titration Reaction Sodium Hydroxide.
From www.numerade.com
SOLVEDmEztion CUNVELAFnment; 1) Predict the titration curve for the Titration Reaction Sodium Hydroxide (3) the titration setup a titration experiment is one where one attempts to determine the concentration of a sample solution by. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The concept and technique of titration. Includes kit list and safety instructions. the above equation works only for. Titration Reaction Sodium Hydroxide.
From www.hotzxgirl.com
Solved Titration Standardization Of Sodium Hydroxide Submit Chegg Hot Titration Reaction Sodium Hydroxide The concept and technique of titration. a titration is an analytical procedure used to determine the concentration of a sample by reacting it with a standard solution. Includes kit list and safety instructions. (3) the titration setup a titration experiment is one where one attempts to determine the concentration of a sample solution by. the above equation. Titration Reaction Sodium Hydroxide.
From www.numerade.com
SOLVED Clearly draw the titration curve for the reaction of this Titration Reaction Sodium Hydroxide Includes kit list and safety instructions. a titration is an analytical procedure used to determine the concentration of a sample by reacting it with a standard solution. (3) the titration setup a titration experiment is one where one attempts to determine the concentration of a sample solution by. the above equation works only for neutralizations in which. Titration Reaction Sodium Hydroxide.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Reaction Sodium Hydroxide (3) the titration setup a titration experiment is one where one attempts to determine the concentration of a sample solution by. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Includes kit list and safety instructions. a titration is an analytical. Titration Reaction Sodium Hydroxide.
From mungfali.com
HCl NaOH Titration Titration Reaction Sodium Hydroxide a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. The concept and technique of titration. a titration is an analytical procedure used to determine the concentration of a sample by reacting it with a standard solution. Includes kit list and safety instructions.. Titration Reaction Sodium Hydroxide.
From www.numerade.com
SOLVED If a solution of acetic acid is titrated with standardized Titration Reaction Sodium Hydroxide Includes kit list and safety instructions. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The concept and technique of titration. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. a titration is an analytical procedure used. Titration Reaction Sodium Hydroxide.
From celjgvoh.blob.core.windows.net
Balanced Equation For The Titration at Barbara Hulbert blog Titration Reaction Sodium Hydroxide (3) the titration setup a titration experiment is one where one attempts to determine the concentration of a sample solution by. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The concept and technique of titration. Includes kit list and safety instructions. a titration is an. Titration Reaction Sodium Hydroxide.
From studylib.net
Experiment (1) Standardization of sodium hydroxide NaOH solution Titration Reaction Sodium Hydroxide the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. (3) the titration setup a titration experiment is one where one attempts to determine the concentration of a sample solution by. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and. Titration Reaction Sodium Hydroxide.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Reaction Sodium Hydroxide a titration is an analytical procedure used to determine the concentration of a sample by reacting it with a standard solution. (3) the titration setup a titration experiment is one where one attempts to determine the concentration of a sample solution by. a titration is an experiment where a volume of a solution of known concentration is. Titration Reaction Sodium Hydroxide.
From printablenemnserioeg.z22.web.core.windows.net
Titration Practical Questions And Answers Titration Reaction Sodium Hydroxide Includes kit list and safety instructions. (3) the titration setup a titration experiment is one where one attempts to determine the concentration of a sample solution by. a titration is an analytical procedure used to determine the concentration of a sample by reacting it with a standard solution. the above equation works only for neutralizations in which. Titration Reaction Sodium Hydroxide.
From www.thinkswap.com
Titration of Sodium Hydroxide with Hydrochloric acid FSC107 General Titration Reaction Sodium Hydroxide The concept and technique of titration. a titration is an analytical procedure used to determine the concentration of a sample by reacting it with a standard solution. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. (3) the titration setup a. Titration Reaction Sodium Hydroxide.
From ivypanda.com
Titration of Acids Standardizing Sodium Hydroxide 500 Words Essay Titration Reaction Sodium Hydroxide the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Includes kit list and safety instructions. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. a titration is an analytical procedure used to determine the concentration of a. Titration Reaction Sodium Hydroxide.
From www.youtube.com
Titration of an unknown acid with a standardized Sodium Hydroxide Titration Reaction Sodium Hydroxide use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The concept and technique of titration. Includes kit list and safety instructions. (3) the titration setup a titration experiment is one where one attempts to determine the concentration of a sample solution by. a titration is an analytical procedure. Titration Reaction Sodium Hydroxide.
From edu.rsc.org
Titrating sodium hydroxide with hydrochloric acid Experiment RSC Titration Reaction Sodium Hydroxide a titration is an analytical procedure used to determine the concentration of a sample by reacting it with a standard solution. The concept and technique of titration. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. a titration is an experiment where a volume of a solution of. Titration Reaction Sodium Hydroxide.
From www.quickmeme.com
Titration memes quickmeme Titration Reaction Sodium Hydroxide Includes kit list and safety instructions. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. a titration is an analytical procedure used to. Titration Reaction Sodium Hydroxide.
From www.youtube.com
Animation Titration Preparation and Standardization of 1M Sodium Titration Reaction Sodium Hydroxide (3) the titration setup a titration experiment is one where one attempts to determine the concentration of a sample solution by. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The concept and technique of titration. a titration is an experiment where a volume of a solution of. Titration Reaction Sodium Hydroxide.
From www.chegg.com
Solved TITRATION ⋅ STANDARDIZATION OF SODIUM HYDROXIDE Titration Reaction Sodium Hydroxide a titration is an analytical procedure used to determine the concentration of a sample by reacting it with a standard solution. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. (3) the titration setup a titration experiment is one where one attempts to determine the concentration. Titration Reaction Sodium Hydroxide.
From www.numerade.com
SOLVED Suppose you are titrating vinegar, which is an acetic acid Titration Reaction Sodium Hydroxide The concept and technique of titration. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Includes kit list and safety instructions. . Titration Reaction Sodium Hydroxide.
From dokumen.tips
(DOCX) · viewUsing Titration to Estimate Rate Law Parameters of the Titration Reaction Sodium Hydroxide a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The concept and technique of titration. Includes kit list and safety instructions. the above. Titration Reaction Sodium Hydroxide.
From edu.rsc.org
Titrating sodium hydroxide with hydrochloric acid Experiment RSC Titration Reaction Sodium Hydroxide the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. a titration is an analytical procedure used to determine the concentration of. Titration Reaction Sodium Hydroxide.
From www.researchgate.net
4. Hydrochloric acid and sodium hydroxide neutralisation curves, [99 Titration Reaction Sodium Hydroxide a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Includes kit list and safety instructions. The concept and technique of titration. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. . Titration Reaction Sodium Hydroxide.
From sciencestruck.com
Titration of Sulfuric Acid and Sodium Hydroxide Science Struck Titration Reaction Sodium Hydroxide Includes kit list and safety instructions. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The concept and technique of titration. (3) the. Titration Reaction Sodium Hydroxide.
From studylib.net
Titration of Hydrochloric Acid with Sodium Hydroxide Titration Reaction Sodium Hydroxide (3) the titration setup a titration experiment is one where one attempts to determine the concentration of a sample solution by. The concept and technique of titration. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. the above equation works only. Titration Reaction Sodium Hydroxide.
From www.studocu.com
Experiment no 1 Determination of sodium carbonate & hydroxide in a Titration Reaction Sodium Hydroxide use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Includes kit list and safety instructions. The concept and technique of titration. a titration is an experiment where a. Titration Reaction Sodium Hydroxide.
From www.chegg.com
Chemistry Archive May 08, 2017 Titration Reaction Sodium Hydroxide a titration is an analytical procedure used to determine the concentration of a sample by reacting it with a standard solution. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The concept and technique of titration. the above equation works only for neutralizations in which there is a. Titration Reaction Sodium Hydroxide.
From dedewall9.netlify.app
Hydrochloric Acid And Sodium Hydroxide Balanced Equation Dede Wallq Titration Reaction Sodium Hydroxide The concept and technique of titration. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Includes kit list and safety instructions. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. . Titration Reaction Sodium Hydroxide.