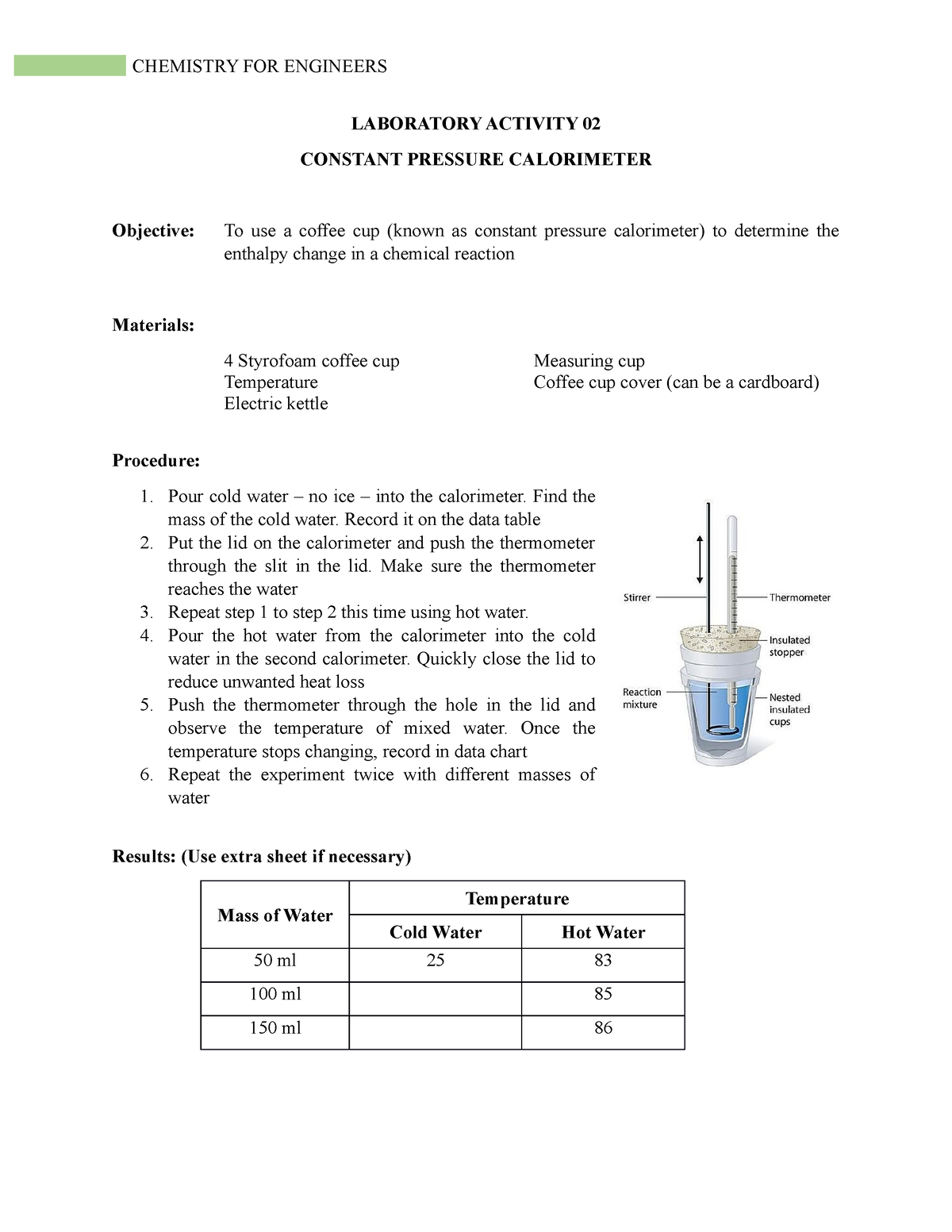
Calorimeter Constant Pressure . This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: The enthalpy changes that accompany. Using this type of calorimeter, you can measure enthalpy change during. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). It's usually made of styrofoam.
from www.studocu.com
This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). The enthalpy changes that accompany. Using this type of calorimeter, you can measure enthalpy change during. It's usually made of styrofoam.
Lab02Calorimetry Lectures and activities LABORATORY ACTIVITY 02
Calorimeter Constant Pressure Using this type of calorimeter, you can measure enthalpy change during. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: The enthalpy changes that accompany. It's usually made of styrofoam. Using this type of calorimeter, you can measure enthalpy change during.
From www.slideserve.com
PPT Thermochemistry 1 PowerPoint Presentation, free download ID Calorimeter Constant Pressure This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: The enthalpy changes that accompany. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). Using this type of calorimeter, you can measure. Calorimeter Constant Pressure.
From www.youtube.com
Constantpressure calorimetry Thermodynamics AP Chemistry Khan Calorimeter Constant Pressure It's usually made of styrofoam. This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: Using this type of calorimeter, you can measure enthalpy change during. The enthalpy changes that accompany. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy. Calorimeter Constant Pressure.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID3207088 Calorimeter Constant Pressure This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: It's usually made of styrofoam. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). The enthalpy changes that accompany. Using this type. Calorimeter Constant Pressure.
From www.slideserve.com
PPT AP Chemistry Unit 7 Thermodynamics PowerPoint Presentation Calorimeter Constant Pressure It's usually made of styrofoam. The enthalpy changes that accompany. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). Using this type of calorimeter, you can measure enthalpy change during. This means that the amount of heat produced or consumed in the reaction equals the amount. Calorimeter Constant Pressure.
From www.youtube.com
1A 6.7 ConstantPressure Calorimetry YouTube Calorimeter Constant Pressure It's usually made of styrofoam. This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: Using this type of calorimeter, you can measure enthalpy change during. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to. Calorimeter Constant Pressure.
From www.slideserve.com
PPT Chemistry 122 PowerPoint Presentation, free download ID6556317 Calorimeter Constant Pressure The enthalpy changes that accompany. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). Using this type of calorimeter, you can measure enthalpy change during. It's usually made of styrofoam. This means that the amount of heat produced or consumed in the reaction equals the amount. Calorimeter Constant Pressure.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Calorimeter Constant Pressure This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: It's usually made of styrofoam. Using this type of calorimeter, you can measure enthalpy change during. The enthalpy changes that accompany. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy. Calorimeter Constant Pressure.
From www.youtube.com
How to find calorimeter constant YouTube Calorimeter Constant Pressure The enthalpy changes that accompany. This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: It's usually made of styrofoam. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). Using this type. Calorimeter Constant Pressure.
From www.slideserve.com
PPT Thermochemistry 1 PowerPoint Presentation, free download ID Calorimeter Constant Pressure Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). The enthalpy changes that accompany. Using this type of calorimeter, you can measure enthalpy change during. This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost. Calorimeter Constant Pressure.
From www.studocu.com
Lab02Calorimetry Lectures and activities LABORATORY ACTIVITY 02 Calorimeter Constant Pressure This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: The enthalpy changes that accompany. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). Using this type of calorimeter, you can measure. Calorimeter Constant Pressure.
From www.w3schools.blog
Cp, Cv calorimetry W3schools Calorimeter Constant Pressure It's usually made of styrofoam. Using this type of calorimeter, you can measure enthalpy change during. This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: The enthalpy changes that accompany. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy. Calorimeter Constant Pressure.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Calorimeter Constant Pressure Using this type of calorimeter, you can measure enthalpy change during. It's usually made of styrofoam. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost. Calorimeter Constant Pressure.
From www.pearson.com
Chapter 09 16 Constant Pressure Calorimetry (coffee cup) Channels Calorimeter Constant Pressure The enthalpy changes that accompany. Using this type of calorimeter, you can measure enthalpy change during. This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to. Calorimeter Constant Pressure.
From app.jove.com
Constant Pressure Calorimeter/ Coffee Cup Calorimeter Chemistry JoVe Calorimeter Constant Pressure It's usually made of styrofoam. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). Using this type of calorimeter, you can measure enthalpy change during. This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost. Calorimeter Constant Pressure.
From www.slideserve.com
PPT Constant Pressure Calorimetry Another Example PowerPoint Calorimeter Constant Pressure The enthalpy changes that accompany. This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: Using this type of calorimeter, you can measure enthalpy change during. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to. Calorimeter Constant Pressure.
From www.youtube.com
AP Chemistry Thermochemistry I Part 4 Constant Pressure Calorimetry Calorimeter Constant Pressure Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). It's usually made of styrofoam. This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: The enthalpy changes that accompany. Using this type. Calorimeter Constant Pressure.
From www.youtube.com
Calorimetry at Constant Pressure YouTube Calorimeter Constant Pressure It's usually made of styrofoam. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). The enthalpy changes that accompany. This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: Using this type. Calorimeter Constant Pressure.
From www.youtube.com
Constantpressure Calorimetry YouTube Calorimeter Constant Pressure It's usually made of styrofoam. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: The enthalpy changes that accompany. Using this type. Calorimeter Constant Pressure.
From rumble.com
Calorimetry, Constant Pressure, Thermodynamics Chemistry Calorimeter Constant Pressure This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: The enthalpy changes that accompany. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). Using this type of calorimeter, you can measure. Calorimeter Constant Pressure.
From exomesmxy.blob.core.windows.net
Bomb Calorimeter Constant Pressure at Mamie Jansen blog Calorimeter Constant Pressure The enthalpy changes that accompany. Using this type of calorimeter, you can measure enthalpy change during. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). It's usually made of styrofoam. This means that the amount of heat produced or consumed in the reaction equals the amount. Calorimeter Constant Pressure.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Calorimeter Constant Pressure The enthalpy changes that accompany. This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). It's usually made of styrofoam. Using this type. Calorimeter Constant Pressure.
From www.slideserve.com
PPT Ch 6 Thermochemistry PowerPoint Presentation, free download ID Calorimeter Constant Pressure It's usually made of styrofoam. Using this type of calorimeter, you can measure enthalpy change during. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). The enthalpy changes that accompany. This means that the amount of heat produced or consumed in the reaction equals the amount. Calorimeter Constant Pressure.
From www.studocu.com
ACT 3 Constant Pressure Calorimetry ACTIVITY 3 CONSTANT PRESSURE Calorimeter Constant Pressure Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). Using this type of calorimeter, you can measure enthalpy change during. The enthalpy changes that accompany. It's usually made of styrofoam. This means that the amount of heat produced or consumed in the reaction equals the amount. Calorimeter Constant Pressure.
From www.youtube.com
Calorimeter Constant lab part 1 YouTube Calorimeter Constant Pressure Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). It's usually made of styrofoam. The enthalpy changes that accompany. Using this type of calorimeter, you can measure enthalpy change during. This means that the amount of heat produced or consumed in the reaction equals the amount. Calorimeter Constant Pressure.
From users.highland.edu
Calorimetry Calorimeter Constant Pressure This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: It's usually made of styrofoam. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). The enthalpy changes that accompany. Using this type. Calorimeter Constant Pressure.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimeter Constant Pressure It's usually made of styrofoam. The enthalpy changes that accompany. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: Using this type. Calorimeter Constant Pressure.
From www.youtube.com
6.7 ConstantPressure Calorimetry Measuring ∆Hrxn YouTube Calorimeter Constant Pressure This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: The enthalpy changes that accompany. It's usually made of styrofoam. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). Using this type. Calorimeter Constant Pressure.
From www.youtube.com
Calorimetry calculation (constant pressure) Example 1 YouTube Calorimeter Constant Pressure Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). It's usually made of styrofoam. The enthalpy changes that accompany. This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: Using this type. Calorimeter Constant Pressure.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Calorimeter Constant Pressure It's usually made of styrofoam. Using this type of calorimeter, you can measure enthalpy change during. The enthalpy changes that accompany. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). This means that the amount of heat produced or consumed in the reaction equals the amount. Calorimeter Constant Pressure.
From www.researchgate.net
Schematic of constant pressure calorimeter for APW classification Calorimeter Constant Pressure Using this type of calorimeter, you can measure enthalpy change during. The enthalpy changes that accompany. It's usually made of styrofoam. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). This means that the amount of heat produced or consumed in the reaction equals the amount. Calorimeter Constant Pressure.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimeter Constant Pressure The enthalpy changes that accompany. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: It's usually made of styrofoam. Using this type. Calorimeter Constant Pressure.
From www.youtube.com
Chapter 09 16 Constant Pressure Calorimetry (coffee cup) YouTube Calorimeter Constant Pressure It's usually made of styrofoam. The enthalpy changes that accompany. This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: Using this type of calorimeter, you can measure enthalpy change during. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy. Calorimeter Constant Pressure.
From saylordotorg.github.io
Calorimetry Calorimeter Constant Pressure This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). It's usually made of styrofoam. Using this type of calorimeter, you can measure. Calorimeter Constant Pressure.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID3756161 Calorimeter Constant Pressure This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). Using this type of calorimeter, you can measure enthalpy change during. It's usually. Calorimeter Constant Pressure.
From www.youtube.com
Constant pressure calorimeter AP chemistry Vittle dennis YouTube Calorimeter Constant Pressure It's usually made of styrofoam. This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution: Using this type of calorimeter, you can measure enthalpy change during. The enthalpy changes that accompany. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy. Calorimeter Constant Pressure.