What Is Vapor Pressure And Boiling Point . Vapor pressure is inversely proportional to the boiling point of a liquid. Learn how boiling occurs, how temperature and pressure. Learn how to measure vapor pressure, how it relates to boiling point and. Boiling is the rapid vaporization of a liquid. The web page provides a clear definition of vapor. Learn how vapor pressure is related to intermolecular forces and temperature, and how it affects the boiling point of liquids. See examples, graphs, and exercises on vapor pressure and boiling point. Learn how temperature, intermolecular forces and other factors affect vapor. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. Vapor pressure is the pressure exerted by a vapor in equilibrium with its condensed phases at a given temperature. Learn how vapor pressure and boiling point are inversely proportional to each other. As the vapor pressure is lowered, the boiling point. Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid state in a closed container.
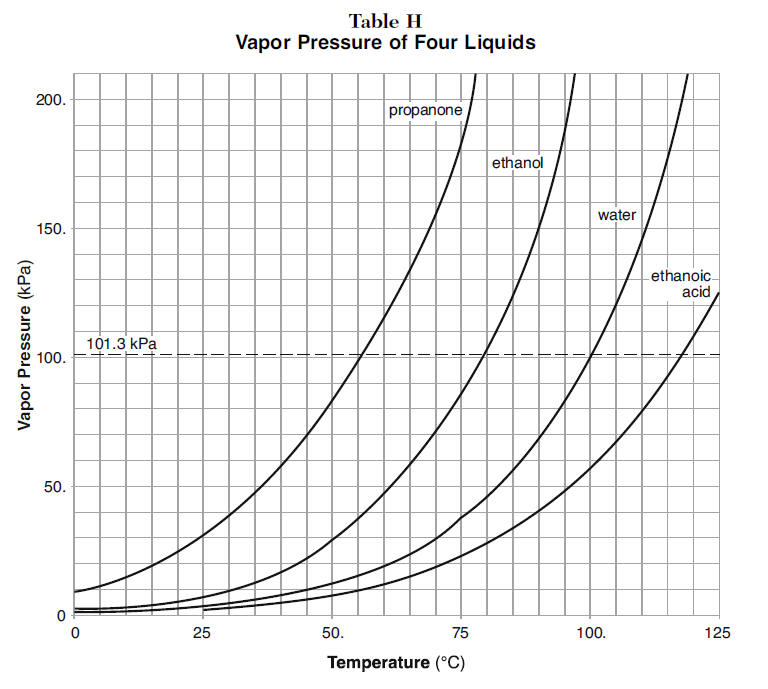
from mr.kentchemistry.com
Vapor pressure is the pressure exerted by a vapor in equilibrium with its condensed phases at a given temperature. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Learn how to measure vapor pressure, how it relates to boiling point and. See examples, graphs, and exercises on vapor pressure and boiling point. Learn how boiling occurs, how temperature and pressure. Boiling is the rapid vaporization of a liquid. The web page provides a clear definition of vapor. Learn how vapor pressure and boiling point are inversely proportional to each other. Learn how temperature, intermolecular forces and other factors affect vapor. As the vapor pressure is lowered, the boiling point.
Determine Boiling Point from Vapor Pressure
What Is Vapor Pressure And Boiling Point Learn how boiling occurs, how temperature and pressure. Boiling is the rapid vaporization of a liquid. Learn how boiling occurs, how temperature and pressure. The web page provides a clear definition of vapor. Learn how to measure vapor pressure, how it relates to boiling point and. Vapor pressure is the pressure exerted by a vapor in equilibrium with its condensed phases at a given temperature. Vapor pressure is inversely proportional to the boiling point of a liquid. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. See examples, graphs, and exercises on vapor pressure and boiling point. Learn how vapor pressure is related to intermolecular forces and temperature, and how it affects the boiling point of liquids. Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid state in a closed container. As the vapor pressure is lowered, the boiling point. Learn how vapor pressure and boiling point are inversely proportional to each other. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. Learn how temperature, intermolecular forces and other factors affect vapor.
From www.youtube.com
Simplest Way To Understand Boiling Point & Vapor Pressure YouTube What Is Vapor Pressure And Boiling Point Learn how to measure vapor pressure, how it relates to boiling point and. Learn how vapor pressure and boiling point are inversely proportional to each other. Learn how boiling occurs, how temperature and pressure. Vapor pressure is inversely proportional to the boiling point of a liquid. The web page provides a clear definition of vapor. Vapor pressure is the pressure. What Is Vapor Pressure And Boiling Point.
From www.slideserve.com
PPT Phases of Matter and Solutions PowerPoint Presentation, free What Is Vapor Pressure And Boiling Point Vapor pressure is inversely proportional to the boiling point of a liquid. Learn how temperature, intermolecular forces and other factors affect vapor. See examples, graphs, and exercises on vapor pressure and boiling point. Learn how vapor pressure and boiling point are inversely proportional to each other. Boiling is the rapid vaporization of a liquid. As the vapor pressure is lowered,. What Is Vapor Pressure And Boiling Point.
From www.worksheetsplanet.com
What is Boiling Point What Is Vapor Pressure And Boiling Point Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. Learn how vapor pressure is related to intermolecular forces and temperature, and how it affects the boiling point of liquids. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Learn how vapor pressure. What Is Vapor Pressure And Boiling Point.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point What Is Vapor Pressure And Boiling Point Vapor pressure is the pressure exerted by a vapor in equilibrium with its condensed phases at a given temperature. Learn how vapor pressure and boiling point are inversely proportional to each other. Boiling is the rapid vaporization of a liquid. Learn how boiling occurs, how temperature and pressure. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium. What Is Vapor Pressure And Boiling Point.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills What Is Vapor Pressure And Boiling Point The web page provides a clear definition of vapor. As the vapor pressure is lowered, the boiling point. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid state in a closed container. Learn how boiling occurs, how temperature and. What Is Vapor Pressure And Boiling Point.
From www.youtube.com
Boiling point and external pressure YouTube What Is Vapor Pressure And Boiling Point Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. Boiling is the rapid vaporization of a liquid. Learn how temperature, intermolecular forces and other factors affect vapor. Learn how vapor pressure is related to intermolecular forces and temperature, and how it affects the boiling point of liquids. The web page provides a clear. What Is Vapor Pressure And Boiling Point.
From www.slideserve.com
PPT Aim What is the relationship between vapor pressure and boiling What Is Vapor Pressure And Boiling Point Boiling is the rapid vaporization of a liquid. Vapor pressure is inversely proportional to the boiling point of a liquid. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. See examples, graphs, and exercises on vapor pressure and boiling point. Vapor pressure is the equilibrium pressure of a vapor above its liquid or. What Is Vapor Pressure And Boiling Point.
From www.slideserve.com
PPT Vapor Pressure and Boiling PowerPoint Presentation, free download What Is Vapor Pressure And Boiling Point Vapor pressure is the pressure exerted by a vapor in equilibrium with its condensed phases at a given temperature. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Learn how vapor pressure and boiling point are inversely proportional to each other. Vapor pressure is the equilibrium pressure of a. What Is Vapor Pressure And Boiling Point.
From mungfali.com
Vapor Pressure And Boiling Point Relationship What Is Vapor Pressure And Boiling Point Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. Learn how boiling occurs, how temperature and pressure. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. As the vapor pressure is lowered, the boiling point. Learn how vapor pressure and boiling point. What Is Vapor Pressure And Boiling Point.
From exoyxxesq.blob.core.windows.net
Evaporation And Atmospheric Pressure Relationship at Darlene Prichard blog What Is Vapor Pressure And Boiling Point Learn how vapor pressure and boiling point are inversely proportional to each other. See examples, graphs, and exercises on vapor pressure and boiling point. Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid state in a closed container. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. Learn. What Is Vapor Pressure And Boiling Point.
From www.youtube.com
Vapour Pressure and Boiling Point Relationship Vapour Pressure vs What Is Vapor Pressure And Boiling Point Learn how vapor pressure is related to intermolecular forces and temperature, and how it affects the boiling point of liquids. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. As the vapor pressure is lowered, the boiling point. Boiling is the process by which a liquid turns into a vapor when it is. What Is Vapor Pressure And Boiling Point.
From www.researchgate.net
The vapor pressure of ethanol vs. the normal boilingpoint temperature What Is Vapor Pressure And Boiling Point As the vapor pressure is lowered, the boiling point. Learn how vapor pressure is related to intermolecular forces and temperature, and how it affects the boiling point of liquids. Learn how boiling occurs, how temperature and pressure. The web page provides a clear definition of vapor. Learn how vapor pressure and boiling point are inversely proportional to each other. Learn. What Is Vapor Pressure And Boiling Point.
From www.slideserve.com
PPT Aim What is the relationship between vapor pressure and boiling What Is Vapor Pressure And Boiling Point As the vapor pressure is lowered, the boiling point. The web page provides a clear definition of vapor. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. Boiling is the rapid vaporization of a liquid. Learn how vapor pressure and boiling point are inversely proportional to each other. See examples, graphs, and exercises. What Is Vapor Pressure And Boiling Point.
From wirepartfrontwards.z14.web.core.windows.net
Phase Diagram Normal Boiling Point What Is Vapor Pressure And Boiling Point Learn how to measure vapor pressure, how it relates to boiling point and. Learn how vapor pressure is related to intermolecular forces and temperature, and how it affects the boiling point of liquids. Learn how temperature, intermolecular forces and other factors affect vapor. Boiling is the process by which a liquid turns into a vapor when it is heated to. What Is Vapor Pressure And Boiling Point.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It What Is Vapor Pressure And Boiling Point Boiling is the rapid vaporization of a liquid. Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid state in a closed container. The web page provides a clear definition of vapor. Learn how boiling occurs, how temperature and pressure. As the vapor pressure is lowered, the boiling point. Learn how temperature, intermolecular forces and other. What Is Vapor Pressure And Boiling Point.
From www.slideserve.com
PPT Vapor Pressure and Boiling PowerPoint Presentation, free download What Is Vapor Pressure And Boiling Point Learn how temperature, intermolecular forces and other factors affect vapor. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. See examples, graphs, and exercises on vapor pressure and boiling point. Learn how boiling occurs, how temperature and pressure. As the vapor pressure is lowered, the boiling point. Vapor pressure is inversely proportional to. What Is Vapor Pressure And Boiling Point.
From www.pinterest.co.uk
Vapour Pressure and Boiling point Vapor, Boiling point, Chemistry class What Is Vapor Pressure And Boiling Point Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Learn how temperature, intermolecular forces and other factors affect vapor. Boiling is the rapid vaporization of a liquid. See examples, graphs, and exercises on vapor pressure and boiling point. Learn how vapor pressure and boiling point are inversely proportional to. What Is Vapor Pressure And Boiling Point.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 What Is Vapor Pressure And Boiling Point Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Boiling is the rapid vaporization of a liquid. As the vapor pressure is lowered, the boiling point. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. See examples, graphs, and exercises on vapor. What Is Vapor Pressure And Boiling Point.
From www.youtube.com
Vapor Pressure Normal Boiling Point & Clausius Clapeyron Equation What Is Vapor Pressure And Boiling Point Vapor pressure is inversely proportional to the boiling point of a liquid. Learn how vapor pressure is related to intermolecular forces and temperature, and how it affects the boiling point of liquids. As the vapor pressure is lowered, the boiling point. See examples, graphs, and exercises on vapor pressure and boiling point. Learn how vapor pressure and boiling point are. What Is Vapor Pressure And Boiling Point.
From mungfali.com
Water Boiling Point Pressure Chart What Is Vapor Pressure And Boiling Point Learn how to measure vapor pressure, how it relates to boiling point and. See examples, graphs, and exercises on vapor pressure and boiling point. Learn how vapor pressure is related to intermolecular forces and temperature, and how it affects the boiling point of liquids. Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid state in. What Is Vapor Pressure And Boiling Point.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition What Is Vapor Pressure And Boiling Point Vapor pressure is the pressure exerted by a vapor in equilibrium with its condensed phases at a given temperature. The web page provides a clear definition of vapor. Vapor pressure is inversely proportional to the boiling point of a liquid. Learn how boiling occurs, how temperature and pressure. Learn how vapor pressure is related to intermolecular forces and temperature, and. What Is Vapor Pressure And Boiling Point.
From www.youtube.com
Vapor pressure and boiling point as Colligative properties Real What Is Vapor Pressure And Boiling Point Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Learn how vapor pressure is related to intermolecular forces and temperature, and how it affects the boiling point of liquids. Learn how boiling occurs, how temperature and pressure. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium. What Is Vapor Pressure And Boiling Point.
From mungfali.com
Vapor Pressure And Boiling Point Relationship What Is Vapor Pressure And Boiling Point Boiling is the rapid vaporization of a liquid. Vapor pressure is the pressure exerted by a vapor in equilibrium with its condensed phases at a given temperature. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Learn how vapor pressure is related to intermolecular forces and temperature, and how. What Is Vapor Pressure And Boiling Point.
From www.youtube.com
Vapor Pressure & Boiling Point YouTube What Is Vapor Pressure And Boiling Point Learn how vapor pressure is related to intermolecular forces and temperature, and how it affects the boiling point of liquids. Vapor pressure is inversely proportional to the boiling point of a liquid. Learn how vapor pressure and boiling point are inversely proportional to each other. Learn how to measure vapor pressure, how it relates to boiling point and. Vapor pressure. What Is Vapor Pressure And Boiling Point.
From www.youtube.com
Vapor Pressure and Boiling YouTube What Is Vapor Pressure And Boiling Point Vapor pressure is the pressure exerted by a vapor in equilibrium with its condensed phases at a given temperature. Learn how temperature, intermolecular forces and other factors affect vapor. As the vapor pressure is lowered, the boiling point. Learn how vapor pressure and boiling point are inversely proportional to each other. Vapor pressure is inversely proportional to the boiling point. What Is Vapor Pressure And Boiling Point.
From www.youtube.com
Vapor Pressure and Boiling YouTube What Is Vapor Pressure And Boiling Point As the vapor pressure is lowered, the boiling point. Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid state in a closed container. The web page provides a clear definition of vapor. Vapor pressure is the pressure exerted by a vapor in equilibrium with its condensed phases at a given temperature. Learn how vapor pressure. What Is Vapor Pressure And Boiling Point.
From mungfali.com
Vapor Pressure And Boiling Point Relationship What Is Vapor Pressure And Boiling Point Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. As the vapor pressure is lowered, the boiling point. The web page provides a clear definition of vapor. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Learn how boiling occurs, how temperature. What Is Vapor Pressure And Boiling Point.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube What Is Vapor Pressure And Boiling Point Learn how temperature, intermolecular forces and other factors affect vapor. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Learn how boiling occurs, how temperature and pressure. Learn how vapor pressure is related. What Is Vapor Pressure And Boiling Point.
From www.slideserve.com
PPT Vapor Pressure and Boiling PowerPoint Presentation, free download What Is Vapor Pressure And Boiling Point Learn how to measure vapor pressure, how it relates to boiling point and. Boiling is the rapid vaporization of a liquid. Vapor pressure is inversely proportional to the boiling point of a liquid. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Learn how vapor pressure and boiling point. What Is Vapor Pressure And Boiling Point.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is Vapor Pressure And Boiling Point Learn how to measure vapor pressure, how it relates to boiling point and. Boiling is the rapid vaporization of a liquid. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Learn how boiling. What Is Vapor Pressure And Boiling Point.
From www.slideserve.com
PPT Energy, Temperature, Phase Changes PowerPoint What Is Vapor Pressure And Boiling Point Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid state in a closed container. Vapor pressure is inversely proportional to the boiling point of a liquid. The web page provides a clear definition of vapor. Boiling is the rapid vaporization of a liquid. Learn how vapor pressure and boiling point are inversely proportional to each. What Is Vapor Pressure And Boiling Point.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Is Vapor Pressure And Boiling Point Learn how temperature, intermolecular forces and other factors affect vapor. Learn how vapor pressure is related to intermolecular forces and temperature, and how it affects the boiling point of liquids. Vapor pressure is the pressure exerted by a vapor in equilibrium with its condensed phases at a given temperature. Boiling is the rapid vaporization of a liquid. As the vapor. What Is Vapor Pressure And Boiling Point.
From socratic.org
What is the relation between critical temperature and boiling point or What Is Vapor Pressure And Boiling Point Learn how vapor pressure and boiling point are inversely proportional to each other. See examples, graphs, and exercises on vapor pressure and boiling point. Learn how to measure vapor pressure, how it relates to boiling point and. Learn how boiling occurs, how temperature and pressure. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given. What Is Vapor Pressure And Boiling Point.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 What Is Vapor Pressure And Boiling Point Learn how vapor pressure is related to intermolecular forces and temperature, and how it affects the boiling point of liquids. Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium at a given temperature. The web page provides a clear definition of vapor. Learn how vapor pressure and boiling point are inversely proportional to each other. Vapor pressure. What Is Vapor Pressure And Boiling Point.
From mr.kentchemistry.com
Determine Boiling Point from Vapor Pressure What Is Vapor Pressure And Boiling Point As the vapor pressure is lowered, the boiling point. Vapor pressure is inversely proportional to the boiling point of a liquid. Learn how to measure vapor pressure, how it relates to boiling point and. Boiling is the rapid vaporization of a liquid. Learn how vapor pressure and boiling point are inversely proportional to each other. Learn how temperature, intermolecular forces. What Is Vapor Pressure And Boiling Point.