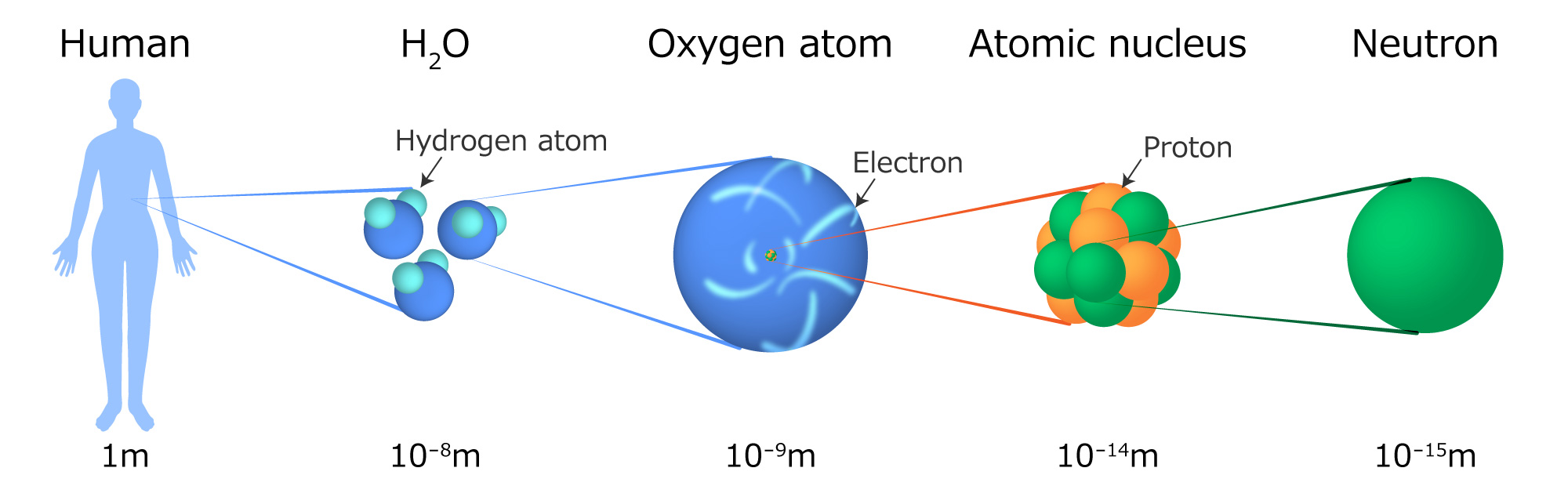
Why Do Atoms Need Neutrons . An atom typically consists of electrons, protons, and neutrons. Electrons are negatively charged and participate in bonding to stabilize the atom. The nucleus of an atom consists of bound protons and neutrons (nucleons). Protons have a positive electrical charge of one (+. The negatively charged electrons are attracted to the positively charged protons and fall around the nucleus, much like a satellite is attracted to the gravity of the earth. If there are many atoms of an element that are isotopes, the average atomic. This is a tiny, dense region at the center of the atom. Protons are found in the nucleus of the atom. Conversely, protons are positively charged. Almost all the atoms in the core turn into neutrons, hence why we call the result a neutron star. An atom consists of electrons, protons, neutrons. Protons are positively charged and electrons are equally negatively charged. Neutron numbers are able to change the mass of atoms, because they weigh about as much as a proton and electron together.
from ar.inspiredpencil.com
The nucleus of an atom consists of bound protons and neutrons (nucleons). Protons are positively charged and electrons are equally negatively charged. Neutron numbers are able to change the mass of atoms, because they weigh about as much as a proton and electron together. Conversely, protons are positively charged. This is a tiny, dense region at the center of the atom. The negatively charged electrons are attracted to the positively charged protons and fall around the nucleus, much like a satellite is attracted to the gravity of the earth. Protons are found in the nucleus of the atom. Protons have a positive electrical charge of one (+. An atom consists of electrons, protons, neutrons. If there are many atoms of an element that are isotopes, the average atomic.
Neutrons
Why Do Atoms Need Neutrons Electrons are negatively charged and participate in bonding to stabilize the atom. Almost all the atoms in the core turn into neutrons, hence why we call the result a neutron star. Protons are found in the nucleus of the atom. Conversely, protons are positively charged. The nucleus of an atom consists of bound protons and neutrons (nucleons). Protons are positively charged and electrons are equally negatively charged. An atom typically consists of electrons, protons, and neutrons. If there are many atoms of an element that are isotopes, the average atomic. An atom consists of electrons, protons, neutrons. Electrons are negatively charged and participate in bonding to stabilize the atom. This is a tiny, dense region at the center of the atom. Protons have a positive electrical charge of one (+. Neutron numbers are able to change the mass of atoms, because they weigh about as much as a proton and electron together. The negatively charged electrons are attracted to the positively charged protons and fall around the nucleus, much like a satellite is attracted to the gravity of the earth.
From slideplayer.com
Today Atomic particles electrons, protons, neutrons ppt download Why Do Atoms Need Neutrons This is a tiny, dense region at the center of the atom. Conversely, protons are positively charged. Protons are positively charged and electrons are equally negatively charged. The negatively charged electrons are attracted to the positively charged protons and fall around the nucleus, much like a satellite is attracted to the gravity of the earth. Electrons are negatively charged and. Why Do Atoms Need Neutrons.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer Why Do Atoms Need Neutrons Almost all the atoms in the core turn into neutrons, hence why we call the result a neutron star. Neutron numbers are able to change the mass of atoms, because they weigh about as much as a proton and electron together. Protons are positively charged and electrons are equally negatively charged. Protons have a positive electrical charge of one (+.. Why Do Atoms Need Neutrons.
From www.nuclear-power.com
Prompt Neutrons Definition & Characteristics Why Do Atoms Need Neutrons Neutron numbers are able to change the mass of atoms, because they weigh about as much as a proton and electron together. Protons have a positive electrical charge of one (+. If there are many atoms of an element that are isotopes, the average atomic. The nucleus of an atom consists of bound protons and neutrons (nucleons). Protons are found. Why Do Atoms Need Neutrons.
From www.expii.com
Neutrons — Structure & Properties Expii Why Do Atoms Need Neutrons The negatively charged electrons are attracted to the positively charged protons and fall around the nucleus, much like a satellite is attracted to the gravity of the earth. An atom consists of electrons, protons, neutrons. Almost all the atoms in the core turn into neutrons, hence why we call the result a neutron star. Conversely, protons are positively charged. Neutron. Why Do Atoms Need Neutrons.
From www.expii.com
Neutrons — Structure & Properties Expii Why Do Atoms Need Neutrons If there are many atoms of an element that are isotopes, the average atomic. The negatively charged electrons are attracted to the positively charged protons and fall around the nucleus, much like a satellite is attracted to the gravity of the earth. Protons have a positive electrical charge of one (+. This is a tiny, dense region at the center. Why Do Atoms Need Neutrons.
From www.hep.ph.bham.ac.uk
Why study particle physics? A brief introduction Why Do Atoms Need Neutrons Electrons are negatively charged and participate in bonding to stabilize the atom. Almost all the atoms in the core turn into neutrons, hence why we call the result a neutron star. Protons are positively charged and electrons are equally negatively charged. This is a tiny, dense region at the center of the atom. Neutron numbers are able to change the. Why Do Atoms Need Neutrons.
From www.expii.com
Neutrons — Structure & Properties Expii Why Do Atoms Need Neutrons Electrons are negatively charged and participate in bonding to stabilize the atom. If there are many atoms of an element that are isotopes, the average atomic. Conversely, protons are positively charged. An atom consists of electrons, protons, neutrons. The nucleus of an atom consists of bound protons and neutrons (nucleons). Protons have a positive electrical charge of one (+. Neutron. Why Do Atoms Need Neutrons.
From ar.inspiredpencil.com
Neutrons Why Do Atoms Need Neutrons Protons are positively charged and electrons are equally negatively charged. Protons have a positive electrical charge of one (+. Electrons are negatively charged and participate in bonding to stabilize the atom. Protons are found in the nucleus of the atom. If there are many atoms of an element that are isotopes, the average atomic. An atom consists of electrons, protons,. Why Do Atoms Need Neutrons.
From slideplayer.com
Why do atoms combine? 1 Atomic Structures ppt download Why Do Atoms Need Neutrons If there are many atoms of an element that are isotopes, the average atomic. An atom consists of electrons, protons, neutrons. Protons are positively charged and electrons are equally negatively charged. Protons are found in the nucleus of the atom. This is a tiny, dense region at the center of the atom. Protons have a positive electrical charge of one. Why Do Atoms Need Neutrons.
From ar.inspiredpencil.com
Neutrons Why Do Atoms Need Neutrons Conversely, protons are positively charged. Almost all the atoms in the core turn into neutrons, hence why we call the result a neutron star. Protons have a positive electrical charge of one (+. Protons are positively charged and electrons are equally negatively charged. An atom typically consists of electrons, protons, and neutrons. Protons are found in the nucleus of the. Why Do Atoms Need Neutrons.
From taylorsciencegeeks.weebly.com
The Role of a Neutron Science News Why Do Atoms Need Neutrons The nucleus of an atom consists of bound protons and neutrons (nucleons). Protons are found in the nucleus of the atom. This is a tiny, dense region at the center of the atom. If there are many atoms of an element that are isotopes, the average atomic. Almost all the atoms in the core turn into neutrons, hence why we. Why Do Atoms Need Neutrons.
From owlcation.com
Atoms, Molecules, and Compounds What's the Difference? Owlcation Why Do Atoms Need Neutrons Protons are found in the nucleus of the atom. Electrons are negatively charged and participate in bonding to stabilize the atom. An atom typically consists of electrons, protons, and neutrons. Protons are positively charged and electrons are equally negatively charged. Protons have a positive electrical charge of one (+. If there are many atoms of an element that are isotopes,. Why Do Atoms Need Neutrons.
From sciencenotes.org
10 Interesting Atom Facts Why Do Atoms Need Neutrons The negatively charged electrons are attracted to the positively charged protons and fall around the nucleus, much like a satellite is attracted to the gravity of the earth. Neutron numbers are able to change the mass of atoms, because they weigh about as much as a proton and electron together. An atom consists of electrons, protons, neutrons. Conversely, protons are. Why Do Atoms Need Neutrons.
From www.broadlearnings.com
Atomic Structure Broad Learnings Why Do Atoms Need Neutrons An atom consists of electrons, protons, neutrons. Protons have a positive electrical charge of one (+. Conversely, protons are positively charged. Protons are positively charged and electrons are equally negatively charged. Neutron numbers are able to change the mass of atoms, because they weigh about as much as a proton and electron together. This is a tiny, dense region at. Why Do Atoms Need Neutrons.
From www.slideserve.com
PPT At the center of every atom is a nucleus containing protons and Why Do Atoms Need Neutrons If there are many atoms of an element that are isotopes, the average atomic. Protons are positively charged and electrons are equally negatively charged. This is a tiny, dense region at the center of the atom. Neutron numbers are able to change the mass of atoms, because they weigh about as much as a proton and electron together. Conversely, protons. Why Do Atoms Need Neutrons.
From www.shalom-education.com
Atomic Structure and Ions GCSE Chemistry Revision Why Do Atoms Need Neutrons Almost all the atoms in the core turn into neutrons, hence why we call the result a neutron star. An atom typically consists of electrons, protons, and neutrons. Conversely, protons are positively charged. The negatively charged electrons are attracted to the positively charged protons and fall around the nucleus, much like a satellite is attracted to the gravity of the. Why Do Atoms Need Neutrons.
From www.youtube.com
Why is an atom electrically neutral YouTube Why Do Atoms Need Neutrons Protons are positively charged and electrons are equally negatively charged. Electrons are negatively charged and participate in bonding to stabilize the atom. Conversely, protons are positively charged. Neutron numbers are able to change the mass of atoms, because they weigh about as much as a proton and electron together. Protons have a positive electrical charge of one (+. An atom. Why Do Atoms Need Neutrons.
From slideplayer.com
Chapter 6 Section ppt download Why Do Atoms Need Neutrons Electrons are negatively charged and participate in bonding to stabilize the atom. The nucleus of an atom consists of bound protons and neutrons (nucleons). The negatively charged electrons are attracted to the positively charged protons and fall around the nucleus, much like a satellite is attracted to the gravity of the earth. If there are many atoms of an element. Why Do Atoms Need Neutrons.
From chemistry.about.com
Basic Model of the Atom Atomic Theory Why Do Atoms Need Neutrons Neutron numbers are able to change the mass of atoms, because they weigh about as much as a proton and electron together. Conversely, protons are positively charged. This is a tiny, dense region at the center of the atom. Protons are positively charged and electrons are equally negatively charged. If there are many atoms of an element that are isotopes,. Why Do Atoms Need Neutrons.
From www.cyberphysics.co.uk
The nucleus Why Do Atoms Need Neutrons Almost all the atoms in the core turn into neutrons, hence why we call the result a neutron star. Electrons are negatively charged and participate in bonding to stabilize the atom. Conversely, protons are positively charged. An atom consists of electrons, protons, neutrons. The negatively charged electrons are attracted to the positively charged protons and fall around the nucleus, much. Why Do Atoms Need Neutrons.
From dxovrqezz.blob.core.windows.net
An Aluminum Atom With 14 Neutrons at Herlinda Wilson blog Why Do Atoms Need Neutrons The negatively charged electrons are attracted to the positively charged protons and fall around the nucleus, much like a satellite is attracted to the gravity of the earth. Conversely, protons are positively charged. The nucleus of an atom consists of bound protons and neutrons (nucleons). An atom consists of electrons, protons, neutrons. This is a tiny, dense region at the. Why Do Atoms Need Neutrons.
From sciencenotes.org
Why Protons and Neutrons Stick Together in the Atomic Nucleus Why Do Atoms Need Neutrons This is a tiny, dense region at the center of the atom. Almost all the atoms in the core turn into neutrons, hence why we call the result a neutron star. Neutron numbers are able to change the mass of atoms, because they weigh about as much as a proton and electron together. The negatively charged electrons are attracted to. Why Do Atoms Need Neutrons.
From wghsjuniorscience.weebly.com
Atomic structure WGHS Junior Science Why Do Atoms Need Neutrons This is a tiny, dense region at the center of the atom. The nucleus of an atom consists of bound protons and neutrons (nucleons). Conversely, protons are positively charged. An atom typically consists of electrons, protons, and neutrons. If there are many atoms of an element that are isotopes, the average atomic. Protons are positively charged and electrons are equally. Why Do Atoms Need Neutrons.
From spmphysics.onlinetuition.com.my
The Composition of the Nucleus SPM Physics Form 4/Form 5 Revision Notes Why Do Atoms Need Neutrons This is a tiny, dense region at the center of the atom. Electrons are negatively charged and participate in bonding to stabilize the atom. Neutron numbers are able to change the mass of atoms, because they weigh about as much as a proton and electron together. Conversely, protons are positively charged. Protons are positively charged and electrons are equally negatively. Why Do Atoms Need Neutrons.
From www.slideserve.com
PPT Atoms and Ions PowerPoint Presentation, free download ID2050882 Why Do Atoms Need Neutrons The nucleus of an atom consists of bound protons and neutrons (nucleons). Neutron numbers are able to change the mass of atoms, because they weigh about as much as a proton and electron together. Protons are positively charged and electrons are equally negatively charged. Almost all the atoms in the core turn into neutrons, hence why we call the result. Why Do Atoms Need Neutrons.
From www.unlimitededu.net
What is a neutron and its charge? Discovery and mass of a neutron Why Do Atoms Need Neutrons Protons are positively charged and electrons are equally negatively charged. Neutron numbers are able to change the mass of atoms, because they weigh about as much as a proton and electron together. Protons have a positive electrical charge of one (+. The nucleus of an atom consists of bound protons and neutrons (nucleons). Conversely, protons are positively charged. Electrons are. Why Do Atoms Need Neutrons.
From www.slideserve.com
PPT The Atom PowerPoint Presentation, free download ID5056341 Why Do Atoms Need Neutrons An atom consists of electrons, protons, neutrons. Protons are positively charged and electrons are equally negatively charged. The negatively charged electrons are attracted to the positively charged protons and fall around the nucleus, much like a satellite is attracted to the gravity of the earth. If there are many atoms of an element that are isotopes, the average atomic. Neutron. Why Do Atoms Need Neutrons.
From www.nagwa.com
Question Video Determining If Two Atoms Are Identical Given the Number Why Do Atoms Need Neutrons Protons are positively charged and electrons are equally negatively charged. This is a tiny, dense region at the center of the atom. The nucleus of an atom consists of bound protons and neutrons (nucleons). Almost all the atoms in the core turn into neutrons, hence why we call the result a neutron star. The negatively charged electrons are attracted to. Why Do Atoms Need Neutrons.
From ar.inspiredpencil.com
Neutrons Why Do Atoms Need Neutrons Protons have a positive electrical charge of one (+. The negatively charged electrons are attracted to the positively charged protons and fall around the nucleus, much like a satellite is attracted to the gravity of the earth. This is a tiny, dense region at the center of the atom. An atom typically consists of electrons, protons, and neutrons. Electrons are. Why Do Atoms Need Neutrons.
From www.wikihow.com
How to Find the Number of Neutrons in an Atom 11 Steps Why Do Atoms Need Neutrons Almost all the atoms in the core turn into neutrons, hence why we call the result a neutron star. The negatively charged electrons are attracted to the positively charged protons and fall around the nucleus, much like a satellite is attracted to the gravity of the earth. Conversely, protons are positively charged. An atom typically consists of electrons, protons, and. Why Do Atoms Need Neutrons.
From ar.inspiredpencil.com
Neutrons Why Do Atoms Need Neutrons The nucleus of an atom consists of bound protons and neutrons (nucleons). Conversely, protons are positively charged. Protons have a positive electrical charge of one (+. Protons are positively charged and electrons are equally negatively charged. The negatively charged electrons are attracted to the positively charged protons and fall around the nucleus, much like a satellite is attracted to the. Why Do Atoms Need Neutrons.
From www.youtube.com
What's Inside An Atom? Protons, Neutrons and Electrons Why Atom Is Why Do Atoms Need Neutrons Conversely, protons are positively charged. This is a tiny, dense region at the center of the atom. Electrons are negatively charged and participate in bonding to stabilize the atom. Almost all the atoms in the core turn into neutrons, hence why we call the result a neutron star. Neutron numbers are able to change the mass of atoms, because they. Why Do Atoms Need Neutrons.
From www.expii.com
Atoms — Definition & Overview Expii Why Do Atoms Need Neutrons This is a tiny, dense region at the center of the atom. Protons are positively charged and electrons are equally negatively charged. Conversely, protons are positively charged. The negatively charged electrons are attracted to the positively charged protons and fall around the nucleus, much like a satellite is attracted to the gravity of the earth. An atom typically consists of. Why Do Atoms Need Neutrons.
From www.animalia-life.club
Electrons In An Atom Why Do Atoms Need Neutrons An atom consists of electrons, protons, neutrons. The negatively charged electrons are attracted to the positively charged protons and fall around the nucleus, much like a satellite is attracted to the gravity of the earth. Protons are positively charged and electrons are equally negatively charged. If there are many atoms of an element that are isotopes, the average atomic. An. Why Do Atoms Need Neutrons.
From www.space.com
Neutrons Facts about the influential subatomic particles Space Why Do Atoms Need Neutrons Protons have a positive electrical charge of one (+. Protons are positively charged and electrons are equally negatively charged. Almost all the atoms in the core turn into neutrons, hence why we call the result a neutron star. Neutron numbers are able to change the mass of atoms, because they weigh about as much as a proton and electron together.. Why Do Atoms Need Neutrons.