Standard Heat Of Formation Cl2 . The standard state heat of formation for the elemental form of each atom is zero. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The elemental form of each atom is that with the lowest enthalpy in the standard state. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we will. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard.
from www.numerade.com
The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard state heat of formation for the elemental form of each atom is zero. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The elemental form of each atom is that with the lowest enthalpy in the standard state. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we will.
SOLVED Which is the correct equation corresponding to the standard
Standard Heat Of Formation Cl2 Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. The standard state heat of formation for the elemental form of each atom is zero. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we will. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The elemental form of each atom is that with the lowest enthalpy in the standard state. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.
From www.coursehero.com
[Solved] Which of the substances have a standard heat of formation Standard Heat Of Formation Cl2 The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. If we have values for the appropriate standard enthalpies of formation, we can determine the. Standard Heat Of Formation Cl2.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of Cl(aq), given Standard Heat Of Formation Cl2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard state heat of formation for the elemental form of each atom is zero. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. If. Standard Heat Of Formation Cl2.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Standard Heat Of Formation Cl2 If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we will. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered. Standard Heat Of Formation Cl2.
From www.slideserve.com
PPT Chemistry 17.4 PowerPoint Presentation, free download ID2772524 Standard Heat Of Formation Cl2 The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we will. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h. Standard Heat Of Formation Cl2.
From studylib.net
PowerPoint 5.3 Enthalpies of Formation and Hess's Law Standard Heat Of Formation Cl2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. The standard state heat of formation for the elemental form of each atom is zero.. Standard Heat Of Formation Cl2.
From www.numerade.com
SOLVED The standard enthalpy change for the following reaction is 359 Standard Heat Of Formation Cl2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered. Standard Heat Of Formation Cl2.
From www.thesciencehive.co.uk
Enthalpy Changes — the science hive Standard Heat Of Formation Cl2 Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol). Standard Heat Of Formation Cl2.
From www.slideserve.com
PPT Thermochemistry Chemical Energy PowerPoint Presentation, free Standard Heat Of Formation Cl2 The elemental form of each atom is that with the lowest enthalpy in the standard state. If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we will. The standard state heat of formation for the elemental form of each atom is zero. Standand enthalpies of formation & standard. Standard Heat Of Formation Cl2.
From www.slideserve.com
PPT Standard Heats of Reaction PowerPoint Presentation, free download Standard Heat Of Formation Cl2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The elemental form of each atom is that with the lowest enthalpy in the standard state. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is. Standard Heat Of Formation Cl2.
From www.youtube.com
🔺️H for the reaction, F2 + 2HCl 》2HF +Cl2 is 352.8kj and standard Standard Heat Of Formation Cl2 The elemental form of each atom is that with the lowest enthalpy in the standard state. If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we will. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the. Standard Heat Of Formation Cl2.
From www.slideshare.net
Heat of formation by reactions Standard Heat Of Formation Cl2 If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we will. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation. Standard Heat Of Formation Cl2.
From www.chegg.com
Solved The standard enthalpy change for the following Standard Heat Of Formation Cl2 Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. If we have values for the appropriate standard enthalpies of formation, we can determine the. Standard Heat Of Formation Cl2.
From www.numerade.com
SOLVED Explain why the standard enthalpy of formation () for Cl2 (g Standard Heat Of Formation Cl2 If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we will. The standard state heat of formation for the elemental form of each atom is zero. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change. Standard Heat Of Formation Cl2.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of Cl(aq) given Standard Heat Of Formation Cl2 Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard state heat of formation for the elemental form of each atom is. Standard Heat Of Formation Cl2.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Standard Heat Of Formation Cl2 The standard state heat of formation for the elemental form of each atom is zero. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the. Standard Heat Of Formation Cl2.
From www.numerade.com
SOLVEDThe species which by definition has ZERO standard molar enthalpy Standard Heat Of Formation Cl2 The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we will. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h. Standard Heat Of Formation Cl2.
From www.numerade.com
SOLVED Using standard heats of formation, calculate the standard Standard Heat Of Formation Cl2 The standard state heat of formation for the elemental form of each atom is zero. The elemental form of each atom is that with the lowest enthalpy in the standard state. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy change of formation. Standard Heat Of Formation Cl2.
From www.numerade.com
SOLVEDCalculate the standard enthalpy of formation of PCl5( s) from Standard Heat Of Formation Cl2 The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard state heat of formation for the elemental form of each atom is zero. The elemental form of each atom is that with the lowest enthalpy in the standard state. Standand enthalpies of formation &. Standard Heat Of Formation Cl2.
From slideplayer.com
Chapter 17 Thermochemistry Heat and Chemical Change ppt video Standard Heat Of Formation Cl2 If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we will. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is a measure of the energy released or consumed when one. Standard Heat Of Formation Cl2.
From www.showme.com
Standard heat of formation Science, Chemistry, thermochemistry ShowMe Standard Heat Of Formation Cl2 The standard state heat of formation for the elemental form of each atom is zero. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. Standard Heat Of Formation Cl2.
From www.numerade.com
SOLVED A scientist measures the standard enthalpy change for the Standard Heat Of Formation Cl2 Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. The elemental form of each atom is that with the lowest enthalpy in the standard state. The. Standard Heat Of Formation Cl2.
From slideplayer.com
Heat in Changes of State and Calculating Heat of Reaction ppt download Standard Heat Of Formation Cl2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we will. Standard enthalpy change of formation (data table) these tables include heat of formation data. Standard Heat Of Formation Cl2.
From www.numerade.com
SOLVED Determine the standard enthalpy change of the following Standard Heat Of Formation Cl2 If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we will. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The elemental form of each atom is that with the lowest enthalpy in. Standard Heat Of Formation Cl2.
From www.numerade.com
SOLVEDUse standard enthalpies of formation from Table 7.2 to determine Standard Heat Of Formation Cl2 The elemental form of each atom is that with the lowest enthalpy in the standard state. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is. Standard Heat Of Formation Cl2.
From byjus.com
Bond dissociaton enthalpy of H2, Cl2 and HCl are 434, 242 and 431 kJ Standard Heat Of Formation Cl2 The standard state heat of formation for the elemental form of each atom is zero. The elemental form of each atom is that with the lowest enthalpy in the standard state. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics, the standard. Standard Heat Of Formation Cl2.
From www.numerade.com
SOLVED Which is the correct equation corresponding to the standard Standard Heat Of Formation Cl2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. If we have values for the appropriate standard enthalpies of formation, we can determine the. Standard Heat Of Formation Cl2.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Heat Of Formation Cl2 Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered. Standard Heat Of Formation Cl2.
From www.numerade.com
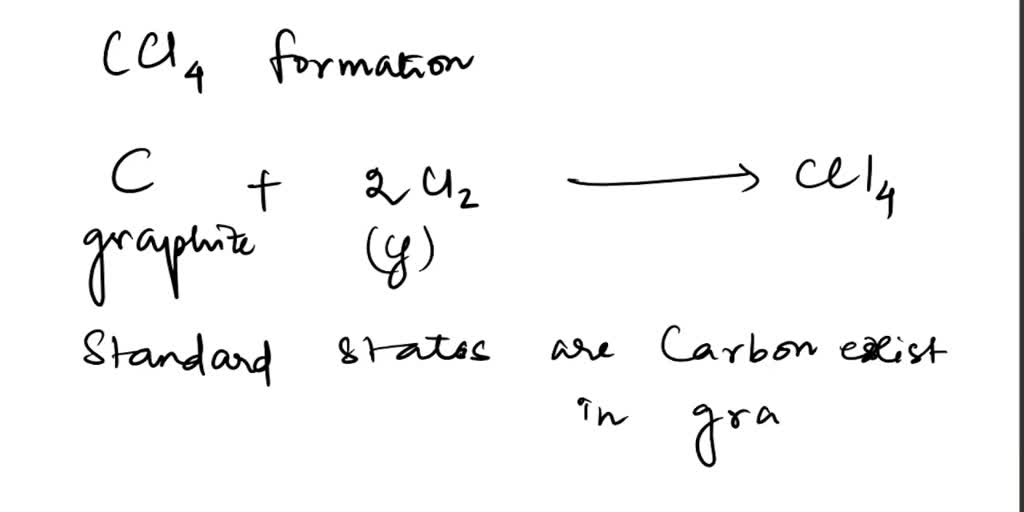
SOLVED 2 Carbon tetrachloride can be formed by reacting chlorine with Standard Heat Of Formation Cl2 If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we will. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard. Standard Heat Of Formation Cl2.
From www.vrogue.co
Standard Enthalpy Of Formation And Standard Free Ener vrogue.co Standard Heat Of Formation Cl2 Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we will. The elemental form of each atom is that with the lowest enthalpy in the standard state.. Standard Heat Of Formation Cl2.
From slideplayer.com
Calculating ΔH using molar heats of formation ppt download Standard Heat Of Formation Cl2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard state heat of formation for the elemental form of each atom is zero. If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we. Standard Heat Of Formation Cl2.
From www.numerade.com
SOLVED 1) Using standard heats of formation, calculate the standard Standard Heat Of Formation Cl2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The elemental form of each atom is that with the lowest enthalpy in the standard state.. Standard Heat Of Formation Cl2.
From www.bartleby.com
Answered Homework 3 • Use standard enthalpies of… bartleby Standard Heat Of Formation Cl2 Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The elemental form of each atom is that with the lowest enthalpy in the standard state. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.. Standard Heat Of Formation Cl2.
From kaden-chapter.blogspot.com
Standard Enthalpy Of Formation Table Pdf 30+ Pages Summary [1.8mb Standard Heat Of Formation Cl2 The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h. Standard Heat Of Formation Cl2.
From www.chegg.com
Table 2.1 Specific heat at constant pressure, Standard Heat Of Formation Cl2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy. Standard Heat Of Formation Cl2.
From www.vrogue.co
Standard Enthalpy Of Formation And Standard Free Ener vrogue.co Standard Heat Of Formation Cl2 Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. The standard state heat of formation for the elemental form of each atom is zero. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.. Standard Heat Of Formation Cl2.