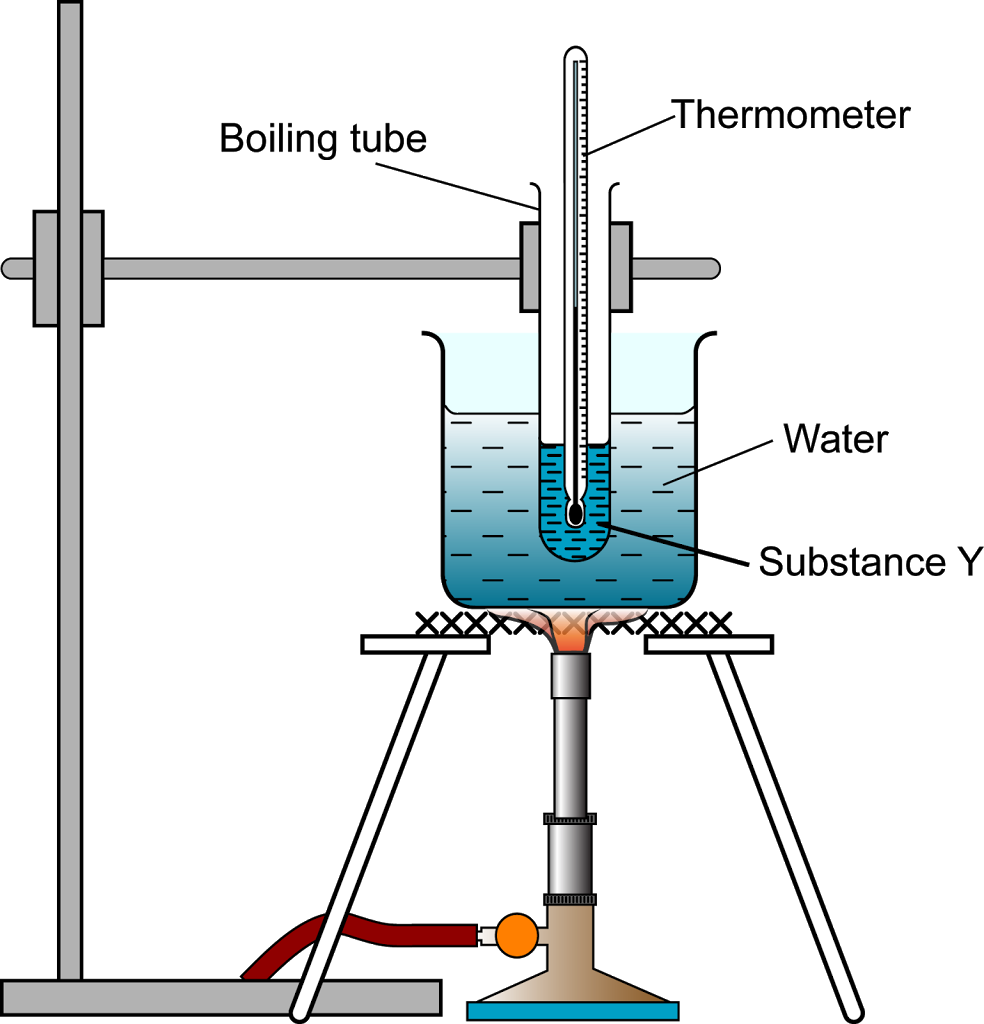
Why Does Oil Heat Water . Oil is thought of as hotter because it can be heated to higher temperatures than. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. understanding water’s specific heat is crucial for designing efficient heating and cooling artificial systems like car. Water is polar because of the bonds between oxygen and hydrogen are polar. the heat capacity of oil is about half that of water. the lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at. the defining equation is δt = δq/c where δt is the change in temperature, δq is the amount of added heat, and c is.
from spmchemistry.blog.onlinetuition.com.my
Water is polar because of the bonds between oxygen and hydrogen are polar. the heat capacity of oil is about half that of water. Oil is thought of as hotter because it can be heated to higher temperatures than. the defining equation is δt = δq/c where δt is the change in temperature, δq is the amount of added heat, and c is. the lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. understanding water’s specific heat is crucial for designing efficient heating and cooling artificial systems like car.
Three States of Matter Structured Question 4 SPM Chemistry
Why Does Oil Heat Water the lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at. the heat capacity of oil is about half that of water. the lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at. Oil is thought of as hotter because it can be heated to higher temperatures than. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. understanding water’s specific heat is crucial for designing efficient heating and cooling artificial systems like car. Water is polar because of the bonds between oxygen and hydrogen are polar. the defining equation is δt = δq/c where δt is the change in temperature, δq is the amount of added heat, and c is.
From inspectapedia.com
Hot water temperature setting, controls, improvement how to Safely Why Does Oil Heat Water Water is polar because of the bonds between oxygen and hydrogen are polar. the lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. understanding water’s. Why Does Oil Heat Water.
From animalia-life.club
Convection Currents In A Room Why Does Oil Heat Water Water is polar because of the bonds between oxygen and hydrogen are polar. the lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at. Oil is thought of as hotter because it can be heated to higher temperatures than. the heat capacity of oil is about half. Why Does Oil Heat Water.
From www.jagranjosh.com
Why Oil and Water does not mix together? Why Does Oil Heat Water understanding water’s specific heat is crucial for designing efficient heating and cooling artificial systems like car. the defining equation is δt = δq/c where δt is the change in temperature, δq is the amount of added heat, and c is. oil boils at a higher temperature than water which means that when water is poured into boiling. Why Does Oil Heat Water.
From slidesharetrick.blogspot.com
Water In Oil Emulsion Diagram slidesharetrick Why Does Oil Heat Water Water is polar because of the bonds between oxygen and hydrogen are polar. understanding water’s specific heat is crucial for designing efficient heating and cooling artificial systems like car. the heat capacity of oil is about half that of water. oil boils at a higher temperature than water which means that when water is poured into boiling. Why Does Oil Heat Water.
From www.shalom-education.com
Conduction and Convection Shalom Education Why Does Oil Heat Water the lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. understanding water’s specific heat is crucial for designing efficient heating and cooling artificial systems like. Why Does Oil Heat Water.
From www.youtube.com
Boiling point of oil is greater than water ? YouTube Why Does Oil Heat Water the defining equation is δt = δq/c where δt is the change in temperature, δq is the amount of added heat, and c is. understanding water’s specific heat is crucial for designing efficient heating and cooling artificial systems like car. oil boils at a higher temperature than water which means that when water is poured into boiling. Why Does Oil Heat Water.
From geoedu.weebly.com
Topic 4 Myths of mantle convection 2 Manifestation of Heat and Onset Why Does Oil Heat Water the heat capacity of oil is about half that of water. the lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at. the defining equation is δt = δq/c where δt is the change in temperature, δq is the amount of added heat, and c is.. Why Does Oil Heat Water.
From inspectapedia.com
oil fired hot water heaters, inspection, diagnosis, repair, replacement Why Does Oil Heat Water the heat capacity of oil is about half that of water. the defining equation is δt = δq/c where δt is the change in temperature, δq is the amount of added heat, and c is. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. Water is. Why Does Oil Heat Water.
From brainly.com
2) According to the article, oil can get a lot hotter than water and Why Does Oil Heat Water the lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at. understanding water’s specific heat is crucial for designing efficient heating and cooling artificial systems like car. Oil is thought of as hotter because it can be heated to higher temperatures than. Water is polar because of. Why Does Oil Heat Water.
From www.tec-science.com
Specific latent heat of condensation tecscience Why Does Oil Heat Water the defining equation is δt = δq/c where δt is the change in temperature, δq is the amount of added heat, and c is. the lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at. oil boils at a higher temperature than water which means that. Why Does Oil Heat Water.
From waterheatertimer.org
How does a boiler work Why Does Oil Heat Water the heat capacity of oil is about half that of water. Oil is thought of as hotter because it can be heated to higher temperatures than. the defining equation is δt = δq/c where δt is the change in temperature, δq is the amount of added heat, and c is. oil boils at a higher temperature than. Why Does Oil Heat Water.
From www.slideserve.com
PPT SPECIFIC HEAT CAPACITY PowerPoint Presentation, free download Why Does Oil Heat Water oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. the defining equation is δt = δq/c where δt is the change in temperature, δq is the amount of added heat, and c is. Water is polar because of the bonds between oxygen and hydrogen are polar. . Why Does Oil Heat Water.
From blog.smarttouchenergy.com
How Your Oil Heating System Works Why Does Oil Heat Water the defining equation is δt = δq/c where δt is the change in temperature, δq is the amount of added heat, and c is. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. Oil is thought of as hotter because it can be heated to higher temperatures. Why Does Oil Heat Water.
From depositphotos.com
Water Oil Dont Mix Infographic Diagram Showing Separated Layers Why Does Oil Heat Water the defining equation is δt = δq/c where δt is the change in temperature, δq is the amount of added heat, and c is. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. Water is polar because of the bonds between oxygen and hydrogen are polar. . Why Does Oil Heat Water.
From www.scifacts.net
Why does oil not mix with water? Science Facts Why Does Oil Heat Water Oil is thought of as hotter because it can be heated to higher temperatures than. the defining equation is δt = δq/c where δt is the change in temperature, δq is the amount of added heat, and c is. oil boils at a higher temperature than water which means that when water is poured into boiling oil it. Why Does Oil Heat Water.
From www.slideserve.com
PPT Unit 4 The Hydrosphere PowerPoint Presentation, free download Why Does Oil Heat Water the heat capacity of oil is about half that of water. the lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. Water is polar because. Why Does Oil Heat Water.
From www.teachoo.com
For any substance, why does the temperature remain constant during the Why Does Oil Heat Water understanding water’s specific heat is crucial for designing efficient heating and cooling artificial systems like car. the lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at. the heat capacity of oil is about half that of water. oil boils at a higher temperature than. Why Does Oil Heat Water.
From mechanic8daasuge.z21.web.core.windows.net
Why Is My Oil Watery Why Does Oil Heat Water the defining equation is δt = δq/c where δt is the change in temperature, δq is the amount of added heat, and c is. understanding water’s specific heat is crucial for designing efficient heating and cooling artificial systems like car. oil boils at a higher temperature than water which means that when water is poured into boiling. Why Does Oil Heat Water.
From www.sciencefacts.net
Heat Transfer Definition, Types, And Examples Why Does Oil Heat Water understanding water’s specific heat is crucial for designing efficient heating and cooling artificial systems like car. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. the heat capacity of oil is about half that of water. the defining equation is δt = δq/c where δt. Why Does Oil Heat Water.
From www.slideserve.com
PPT SPECIFIC HEAT CAPACITY PowerPoint Presentation, free download Why Does Oil Heat Water understanding water’s specific heat is crucial for designing efficient heating and cooling artificial systems like car. the heat capacity of oil is about half that of water. Oil is thought of as hotter because it can be heated to higher temperatures than. the defining equation is δt = δq/c where δt is the change in temperature, δq. Why Does Oil Heat Water.
From www.pirobloc.com
Hot oil heaters and thermal fluids the complete guide Pirobloc Why Does Oil Heat Water the heat capacity of oil is about half that of water. understanding water’s specific heat is crucial for designing efficient heating and cooling artificial systems like car. Water is polar because of the bonds between oxygen and hydrogen are polar. the lower specific heat of oil means that it is easier to heat or cool than water,. Why Does Oil Heat Water.
From smoenergy.com
How Does Oil Heat My Home? SMO Energy Why Does Oil Heat Water Oil is thought of as hotter because it can be heated to higher temperatures than. the lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at. Water is polar because of the bonds between oxygen and hydrogen are polar. the defining equation is δt = δq/c where. Why Does Oil Heat Water.
From www.expii.com
High Specific Heat (Water) — Properties & Examples Expii Why Does Oil Heat Water oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. the lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at. Water is polar because of the bonds between oxygen and hydrogen are polar. understanding water’s. Why Does Oil Heat Water.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Why Does Oil Heat Water Water is polar because of the bonds between oxygen and hydrogen are polar. the heat capacity of oil is about half that of water. understanding water’s specific heat is crucial for designing efficient heating and cooling artificial systems like car. oil boils at a higher temperature than water which means that when water is poured into boiling. Why Does Oil Heat Water.
From exoulkenq.blob.core.windows.net
Does Oil Heat Have A Pilot Light at John Chavarria blog Why Does Oil Heat Water the defining equation is δt = δq/c where δt is the change in temperature, δq is the amount of added heat, and c is. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. Water is polar because of the bonds between oxygen and hydrogen are polar. Oil. Why Does Oil Heat Water.
From vnemart.com.vn
Top 6 why does oil float on water hottest, don't miss Electronic Why Does Oil Heat Water the lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. the defining equation is δt = δq/c where δt is the change in temperature, δq. Why Does Oil Heat Water.
From meddic.jp
heat of condensation meddic Why Does Oil Heat Water oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. the defining equation is δt = δq/c where δt is the change in temperature, δq is the amount of added heat, and c is. Water is polar because of the bonds between oxygen and hydrogen are polar. Oil. Why Does Oil Heat Water.
From wisc.pb.unizin.org
M11Q2 Heating Curves and Phase Diagrams Chem 103/104 Resource Book Why Does Oil Heat Water Oil is thought of as hotter because it can be heated to higher temperatures than. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. the heat capacity of oil is about half that of water. the lower specific heat of oil means that it is easier. Why Does Oil Heat Water.
From www.edusmartzone.com
Why Does Oil Float On Water? All You Want To Know EDU Smart Zone Why Does Oil Heat Water the heat capacity of oil is about half that of water. the lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. Water is polar because. Why Does Oil Heat Water.
From www.slideserve.com
PPT Chapter 2 Aqueous Chemistry PowerPoint Presentation, free Why Does Oil Heat Water oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. the lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at. Water is polar because of the bonds between oxygen and hydrogen are polar. the defining. Why Does Oil Heat Water.
From cooperfuel.com
Heating Oil 3 Things You Don't Need to Worry About Cooper Fuel Why Does Oil Heat Water the lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at. Oil is thought of as hotter because it can be heated to higher temperatures than. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. . Why Does Oil Heat Water.
From sippin.com
oil_water_heater Sippin Energy Products Why Does Oil Heat Water the lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at. understanding water’s specific heat is crucial for designing efficient heating and cooling artificial systems like car. Oil is thought of as hotter because it can be heated to higher temperatures than. oil boils at a. Why Does Oil Heat Water.
From oilheatgurokugi.blogspot.com
Oil Heat Why Does Oil Heat Faster Than Water Why Does Oil Heat Water the heat capacity of oil is about half that of water. understanding water’s specific heat is crucial for designing efficient heating and cooling artificial systems like car. Water is polar because of the bonds between oxygen and hydrogen are polar. the lower specific heat of oil means that it is easier to heat or cool than water,. Why Does Oil Heat Water.
From diagramlibraryclopped.z19.web.core.windows.net
Hot Water Boiler Heating System Diagram Why Does Oil Heat Water Water is polar because of the bonds between oxygen and hydrogen are polar. Oil is thought of as hotter because it can be heated to higher temperatures than. the defining equation is δt = δq/c where δt is the change in temperature, δq is the amount of added heat, and c is. oil boils at a higher temperature. Why Does Oil Heat Water.
From spmchemistry.blog.onlinetuition.com.my
Three States of Matter Structured Question 4 SPM Chemistry Why Does Oil Heat Water Oil is thought of as hotter because it can be heated to higher temperatures than. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. the heat capacity of oil is about half that of water. the defining equation is δt = δq/c where δt is the. Why Does Oil Heat Water.