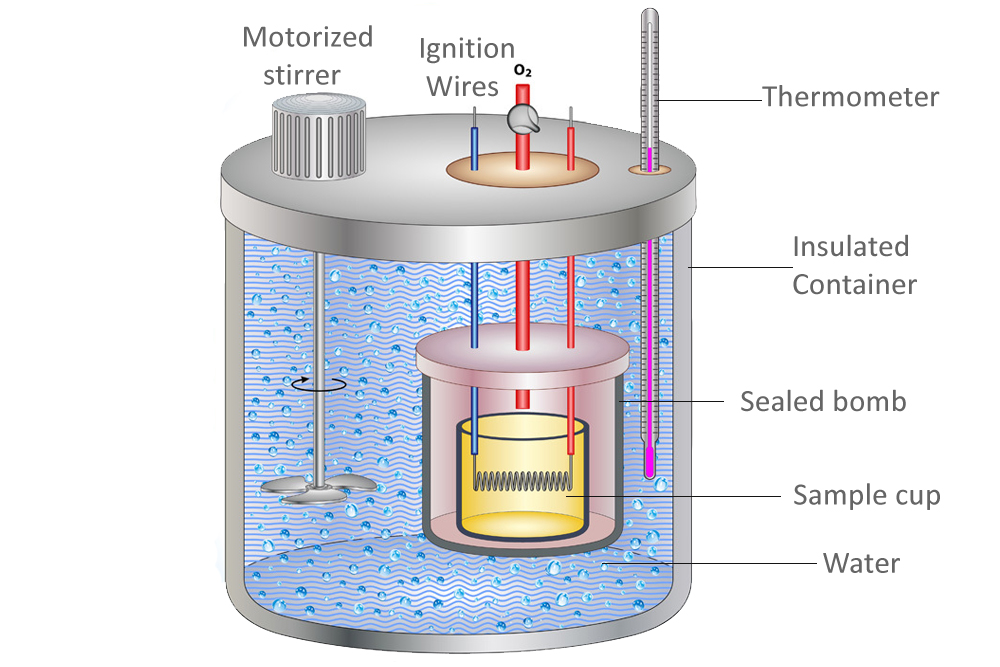
Calorimeter Constant Definition . The calibration gives you a number called the calorimeter constant. What is the calorimeter constant? That is, the calorimeter itself has a heat capacitance. It's the amount of heat energy required to raise the temperature of the calorimeter by. For example, when an exothermic. This usually needs to be measured for each calorimeter, and following the conventions of section 4.2, we. A calorimeter constant (denoted c cal) is a constant that quantifies the heat capacity of a calorimeter. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the. It may be calculated by applying a known amount of.
from www.scienceabc.com
It may be calculated by applying a known amount of. This usually needs to be measured for each calorimeter, and following the conventions of section 4.2, we. That is, the calorimeter itself has a heat capacitance. What is the calorimeter constant? A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. It's the amount of heat energy required to raise the temperature of the calorimeter by. A calorimeter constant (denoted c cal) is a constant that quantifies the heat capacity of a calorimeter. The calibration gives you a number called the calorimeter constant.
Molar Heat Capacity Definition, Formula, Equation, Calculation
Calorimeter Constant Definition The calibration gives you a number called the calorimeter constant. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It's the amount of heat energy required to raise the temperature of the calorimeter by. It may be calculated by applying a known amount of. A calorimeter constant (denoted c cal) is a constant that quantifies the heat capacity of a calorimeter. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. That is, the calorimeter itself has a heat capacitance. What is the calorimeter constant? For example, when an exothermic. The calibration gives you a number called the calorimeter constant. This usually needs to be measured for each calorimeter, and following the conventions of section 4.2, we.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Constant Definition For example, when an exothermic. That is, the calorimeter itself has a heat capacitance. It may be calculated by applying a known amount of. What is the calorimeter constant? It's the amount of heat energy required to raise the temperature of the calorimeter by. A calorimeter constant (denoted c cal) is a constant that quantifies the heat capacity of a. Calorimeter Constant Definition.
From scienceinfo.com
Calorimeter Definition, Types and Uses Calorimeter Constant Definition We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the. What is the calorimeter constant? A calorimeter constant (denoted c cal) is a constant that quantifies the heat capacity of a calorimeter. It may be calculated by applying a known amount of. This usually needs to be. Calorimeter Constant Definition.
From users.highland.edu
Calorimetry Calorimeter Constant Definition We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter constant (denoted c cal) is a constant that quantifies the heat capacity of a calorimeter. The. Calorimeter Constant Definition.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Calorimeter Constant Definition It's the amount of heat energy required to raise the temperature of the calorimeter by. That is, the calorimeter itself has a heat capacitance. What is the calorimeter constant? For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The calibration gives you a number called. Calorimeter Constant Definition.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube Calorimeter Constant Definition That is, the calorimeter itself has a heat capacitance. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The calibration gives you a number called the calorimeter constant. A calorimeter constant (denoted c cal) is a constant that quantifies the heat capacity of a calorimeter. We need to find the. Calorimeter Constant Definition.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID373573 Calorimeter Constant Definition We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the. What is the calorimeter constant? The calibration gives you a number called the calorimeter constant. A calorimeter constant (denoted c cal) is a constant that quantifies the heat capacity of a calorimeter. That is, the calorimeter itself. Calorimeter Constant Definition.
From www.youtube.com
1A 6.7 ConstantPressure Calorimetry YouTube Calorimeter Constant Definition It's the amount of heat energy required to raise the temperature of the calorimeter by. It may be calculated by applying a known amount of. For example, when an exothermic. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. A calorimeter is a device used to measure the amount. Calorimeter Constant Definition.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimeter Constant Definition What is the calorimeter constant? A calorimeter constant (denoted c cal) is a constant that quantifies the heat capacity of a calorimeter. That is, the calorimeter itself has a heat capacitance. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the. The calibration gives you a number. Calorimeter Constant Definition.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimeter Constant Definition A calorimeter constant (denoted c cal) is a constant that quantifies the heat capacity of a calorimeter. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical. Calorimeter Constant Definition.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimeter Constant Definition The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. For example, when an exothermic. That is, the calorimeter itself has a heat capacitance. The calibration gives you a number called the calorimeter constant. It may be calculated by applying a known amount of. It's the amount of heat energy. Calorimeter Constant Definition.
From www.youtube.com
Calorimeter Constant lab part 1 YouTube Calorimeter Constant Definition The calibration gives you a number called the calorimeter constant. A calorimeter constant (denoted c cal) is a constant that quantifies the heat capacity of a calorimeter. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. That is, the calorimeter itself has a heat capacitance.. Calorimeter Constant Definition.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Calorimeter Constant Definition It may be calculated by applying a known amount of. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. What is the calorimeter constant? A calorimeter constant (denoted c cal) is a constant that quantifies the heat capacity of a calorimeter. This usually needs to be measured for each. Calorimeter Constant Definition.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimeter Constant Definition It's the amount of heat energy required to raise the temperature of the calorimeter by. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. A calorimeter constant (denoted c cal) is a constant that quantifies the heat capacity of a calorimeter. A calorimeter is a device used to measure. Calorimeter Constant Definition.
From learningcampusscarf.z13.web.core.windows.net
How To Calculate Heat Of Reaction Calorimetry Calorimeter Constant Definition What is the calorimeter constant? It's the amount of heat energy required to raise the temperature of the calorimeter by. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. That is, the calorimeter itself has a heat capacitance. The calibration gives you a number called the calorimeter constant. We need. Calorimeter Constant Definition.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimeter Constant Definition We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the. The calibration gives you a number called the calorimeter constant. A calorimeter constant (denoted c cal) is a constant that quantifies the heat capacity of a calorimeter. A calorimeter is a device used to measure the amount. Calorimeter Constant Definition.
From exomesmxy.blob.core.windows.net
Bomb Calorimeter Constant Pressure at Mamie Jansen blog Calorimeter Constant Definition We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the. This usually needs to be measured for each calorimeter, and following the conventions of section 4.2, we. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter.. Calorimeter Constant Definition.
From saylordotorg.github.io
Calorimetry Calorimeter Constant Definition We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. This usually needs to be measured for each calorimeter, and following the conventions. Calorimeter Constant Definition.
From exourbxre.blob.core.windows.net
Why Is Calorimeter Constant Important at Waldman blog Calorimeter Constant Definition For example, when an exothermic. A calorimeter constant (denoted c cal) is a constant that quantifies the heat capacity of a calorimeter. What is the calorimeter constant? This usually needs to be measured for each calorimeter, and following the conventions of section 4.2, we. We need to find the difference between the heat lost by the hot water when it. Calorimeter Constant Definition.
From joiksvxlu.blob.core.windows.net
How Does Calorimeter Constant Work at Stacy Cavallo blog Calorimeter Constant Definition The calibration gives you a number called the calorimeter constant. This usually needs to be measured for each calorimeter, and following the conventions of section 4.2, we. It may be calculated by applying a known amount of. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. What is the calorimeter. Calorimeter Constant Definition.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimeter Constant Definition The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. A calorimeter constant (denoted c cal) is a constant that quantifies the heat capacity of a calorimeter. It's the amount of heat energy required to raise the temperature of the calorimeter by. What is the calorimeter constant? That is, the. Calorimeter Constant Definition.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2692866 Calorimeter Constant Definition That is, the calorimeter itself has a heat capacitance. It's the amount of heat energy required to raise the temperature of the calorimeter by. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the. This usually needs to be measured for each calorimeter, and following the conventions. Calorimeter Constant Definition.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Calorimeter Constant Definition For example, when an exothermic. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the. The calibration gives you a number called the calorimeter constant. This usually needs to be measured for each calorimeter, and following the conventions of section 4.2, we. A calorimeter constant (denoted c. Calorimeter Constant Definition.
From dxojkdaez.blob.core.windows.net
Calorimetry Physics Examples at Jeremy Wetmore blog Calorimeter Constant Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. This usually needs to be measured for each calorimeter, and following the conventions of section 4.2, we. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the. A. Calorimeter Constant Definition.
From www.youtube.com
Principle of Calorimetry YouTube Calorimeter Constant Definition The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. It's the amount of heat energy required to raise the temperature of the calorimeter by. The calibration gives you a number called the calorimeter constant. We need to find the difference between the heat lost by the hot water when. Calorimeter Constant Definition.
From joiksvxlu.blob.core.windows.net
How Does Calorimeter Constant Work at Stacy Cavallo blog Calorimeter Constant Definition The calibration gives you a number called the calorimeter constant. What is the calorimeter constant? That is, the calorimeter itself has a heat capacitance. A calorimeter constant (denoted c cal) is a constant that quantifies the heat capacity of a calorimeter. We need to find the difference between the heat lost by the hot water when it droped from 60.0. Calorimeter Constant Definition.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter Constant Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter constant (denoted c cal) is a constant that quantifies the heat capacity of a calorimeter. This usually needs to be measured for each calorimeter, and following the conventions of section 4.2, we. The calorimeter constant, also known as the. Calorimeter Constant Definition.
From joiksvxlu.blob.core.windows.net
How Does Calorimeter Constant Work at Stacy Cavallo blog Calorimeter Constant Definition That is, the calorimeter itself has a heat capacitance. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. For example, when an exothermic. The calibration gives you a number called the calorimeter constant. This usually needs to be measured for each calorimeter, and following the conventions of section 4.2,. Calorimeter Constant Definition.
From www.youtube.com
How to find calorimeter constant YouTube Calorimeter Constant Definition It may be calculated by applying a known amount of. What is the calorimeter constant? The calibration gives you a number called the calorimeter constant. That is, the calorimeter itself has a heat capacitance. It's the amount of heat energy required to raise the temperature of the calorimeter by. A calorimeter constant (denoted c cal) is a constant that quantifies. Calorimeter Constant Definition.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Constant Definition A calorimeter constant (denoted c cal) is a constant that quantifies the heat capacity of a calorimeter. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. What is the calorimeter constant? It's the amount of heat energy required to raise the temperature of the calorimeter by. A calorimeter is. Calorimeter Constant Definition.
From www.youtube.com
Thermochemistry ConstantVolume Calorimeter (Bomb Calorimeter). YouTube Calorimeter Constant Definition The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. It's the amount of heat energy required to raise the temperature of the calorimeter by. It may be calculated by applying a known amount of. For example, when an exothermic. This usually needs to be measured for each calorimeter, and. Calorimeter Constant Definition.
From www.youtube.com
Thermochemistry 5 Determining Calorimeter Constant 6m16s YouTube Calorimeter Constant Definition The calibration gives you a number called the calorimeter constant. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. What is the calorimeter constant? This usually needs to be measured for each calorimeter, and following the conventions of section 4.2, we. A calorimeter is a device used to measure. Calorimeter Constant Definition.
From studylib.net
Calorimetry Calorimeter Constant Definition This usually needs to be measured for each calorimeter, and following the conventions of section 4.2, we. A calorimeter constant (denoted c cal) is a constant that quantifies the heat capacity of a calorimeter. That is, the calorimeter itself has a heat capacitance. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity. Calorimeter Constant Definition.
From www.youtube.com
Calorimetry calculation YouTube Calorimeter Constant Definition It's the amount of heat energy required to raise the temperature of the calorimeter by. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. The calibration gives you a number called the calorimeter constant. It may be calculated by applying a known amount of. A calorimeter is a device. Calorimeter Constant Definition.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Constant Definition What is the calorimeter constant? This usually needs to be measured for each calorimeter, and following the conventions of section 4.2, we. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical. Calorimeter Constant Definition.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimeter Constant Definition That is, the calorimeter itself has a heat capacitance. What is the calorimeter constant? This usually needs to be measured for each calorimeter, and following the conventions of section 4.2, we. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. It may be calculated by applying a known amount. Calorimeter Constant Definition.