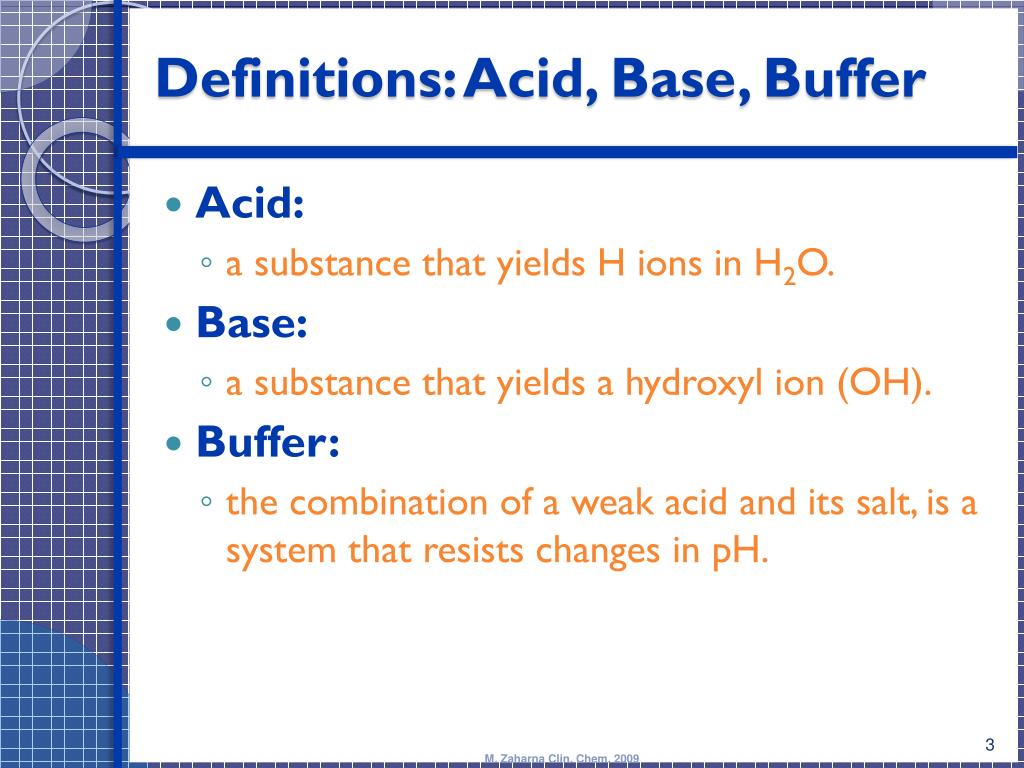
Explain The Buffer System In The Blood . A buffer is a solution that resists sudden changes in ph. Identify the most powerful buffer system in the body. Venous blood carries more co 2 than arterial blood. In this system, gaseous metabolic waste carbon dioxide reacts. Hence, the ph of venous blood is more acid than that of. Introduction to buffer systems of blood: Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Explain the way in which the respiratory system affects blood ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The ph of the blood is maintained between 7.35 and 7.45 by an important buffer system.
from www.slideserve.com
The ph of the blood is maintained between 7.35 and 7.45 by an important buffer system. Hence, the ph of venous blood is more acid than that of. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this system, gaseous metabolic waste carbon dioxide reacts. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. A buffer is a solution that resists sudden changes in ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Introduction to buffer systems of blood: Explain the way in which the respiratory system affects blood ph. Venous blood carries more co 2 than arterial blood.
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615
Explain The Buffer System In The Blood Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. A buffer is a solution that resists sudden changes in ph. Introduction to buffer systems of blood: The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Venous blood carries more co 2 than arterial blood. Explain the way in which the respiratory system affects blood ph. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. Hence, the ph of venous blood is more acid than that of. Identify the most powerful buffer system in the body. In this system, gaseous metabolic waste carbon dioxide reacts. The ph of the blood is maintained between 7.35 and 7.45 by an important buffer system.
From www.slideserve.com
PPT Integrative Physiology PowerPoint Presentation, free download Explain The Buffer System In The Blood Explain the way in which the respiratory system affects blood ph. Identify the most powerful buffer system in the body. Venous blood carries more co 2 than arterial blood. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The buffer systems functioning in. Explain The Buffer System In The Blood.
From www.youtube.com
Buffer in biological system buffer system in blood buffer in eyes Explain The Buffer System In The Blood Explain the way in which the respiratory system affects blood ph. In this system, gaseous metabolic waste carbon dioxide reacts. The ph of the blood is maintained between 7.35 and 7.45 by an important buffer system. Introduction to buffer systems of blood: Hence, the ph of venous blood is more acid than that of. The buffer systems functioning in blood. Explain The Buffer System In The Blood.
From www.slideshare.net
Blood buffer system Explain The Buffer System In The Blood Hence, the ph of venous blood is more acid than that of. Explain the way in which the respiratory system affects blood ph. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate,. Explain The Buffer System In The Blood.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Explain The Buffer System In The Blood Hence, the ph of venous blood is more acid than that of. In this system, gaseous metabolic waste carbon dioxide reacts. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. Venous blood carries more co 2 than arterial blood. The buffer systems functioning. Explain The Buffer System In The Blood.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Explain The Buffer System In The Blood In this system, gaseous metabolic waste carbon dioxide reacts. Explain the way in which the respiratory system affects blood ph. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. Introduction to buffer systems of blood: Hence, the ph of venous blood is more. Explain The Buffer System In The Blood.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Explain The Buffer System In The Blood The ph of the blood is maintained between 7.35 and 7.45 by an important buffer system. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. In this system, gaseous metabolic waste carbon dioxide reacts. Venous blood carries more co 2 than arterial blood.. Explain The Buffer System In The Blood.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 Explain The Buffer System In The Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this system, gaseous metabolic waste carbon dioxide reacts. Venous blood carries more co 2 than arterial blood. Hence, the ph of venous blood is more acid than that of. A buffer is a solution that resists sudden changes in ph. Introduction to. Explain The Buffer System In The Blood.
From www.slideserve.com
PPT Blood Buffers PowerPoint Presentation, free download ID5687657 Explain The Buffer System In The Blood Explain the way in which the respiratory system affects blood ph. Introduction to buffer systems of blood: The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A buffer is a solution that resists sudden changes. Explain The Buffer System In The Blood.
From www.slideserve.com
PPT Physiological system of blood. Functional importance of blood Explain The Buffer System In The Blood A buffer is a solution that resists sudden changes in ph. The ph of the blood is maintained between 7.35 and 7.45 by an important buffer system. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion. Explain The Buffer System In The Blood.
From www.slideshare.net
Buffer system Explain The Buffer System In The Blood Hence, the ph of venous blood is more acid than that of. The ph of the blood is maintained between 7.35 and 7.45 by an important buffer system. In this system, gaseous metabolic waste carbon dioxide reacts. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human blood contains a buffer of. Explain The Buffer System In The Blood.
From beta.learner.org
The Buffer System in the Blood (animation) Annenberg Learner Explain The Buffer System In The Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Identify the most powerful buffer system in the body. Explain the way in which the respiratory system affects blood ph. In this system, gaseous metabolic waste carbon dioxide reacts. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. Explain The Buffer System In The Blood.
From www.slideserve.com
PPT MLAB 2401 Clinical Chemistry Keri BrophyMartinez PowerPoint Explain The Buffer System In The Blood Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this system, gaseous metabolic waste carbon dioxide reacts. Introduction to buffer systems of blood: The buffer. Explain The Buffer System In The Blood.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Explain The Buffer System In The Blood The ph of the blood is maintained between 7.35 and 7.45 by an important buffer system. Explain the way in which the respiratory system affects blood ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Identify the most powerful buffer system in the body. Hence, the ph of venous blood is. Explain The Buffer System In The Blood.
From www.slideshare.net
Buffer in the blood Explain The Buffer System In The Blood Venous blood carries more co 2 than arterial blood. A buffer is a solution that resists sudden changes in ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Introduction to buffer systems of blood: The ph of the blood is maintained between 7.35 and 7.45 by an important buffer system. Explain. Explain The Buffer System In The Blood.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Explain The Buffer System In The Blood Introduction to buffer systems of blood: Explain the way in which the respiratory system affects blood ph. Venous blood carries more co 2 than arterial blood. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A buffer is a solution that resists sudden changes in ph. Human blood contains a buffer of. Explain The Buffer System In The Blood.
From chempedia.info
Buffer systems of the blood Big Chemical Encyclopedia Explain The Buffer System In The Blood Venous blood carries more co 2 than arterial blood. Introduction to buffer systems of blood: Identify the most powerful buffer system in the body. In this system, gaseous metabolic waste carbon dioxide reacts. Hence, the ph of venous blood is more acid than that of. A buffer is a solution that resists sudden changes in ph. Human blood contains a. Explain The Buffer System In The Blood.
From www.youtube.com
1 Regulation of Blood pH By Buffer SystemsAcid Base Balance Explain The Buffer System In The Blood Hence, the ph of venous blood is more acid than that of. The ph of the blood is maintained between 7.35 and 7.45 by an important buffer system. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The buffer systems functioning in blood. Explain The Buffer System In The Blood.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download Explain The Buffer System In The Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph.. Explain The Buffer System In The Blood.
From www.youtube.com
Buffer action in the blood YouTube Explain The Buffer System In The Blood Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A buffer is a solution that resists sudden changes in ph. Introduction to buffer systems of blood:. Explain The Buffer System In The Blood.
From www.slideserve.com
PPT Chapter 8 Acids & Bases PowerPoint Presentation ID1121468 Explain The Buffer System In The Blood In this system, gaseous metabolic waste carbon dioxide reacts. Introduction to buffer systems of blood: Hence, the ph of venous blood is more acid than that of. Explain the way in which the respiratory system affects blood ph. Identify the most powerful buffer system in the body. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate. Explain The Buffer System In The Blood.
From www.slideserve.com
PPT Physiological system of blood. Functional importance of blood Explain The Buffer System In The Blood The ph of the blood is maintained between 7.35 and 7.45 by an important buffer system. Explain the way in which the respiratory system affects blood ph. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The buffer systems functioning in blood plasma. Explain The Buffer System In The Blood.
From www.slideserve.com
PPT General, Organic, and Biochemistry, 7e PowerPoint Presentation Explain The Buffer System In The Blood Explain the way in which the respiratory system affects blood ph. Hence, the ph of venous blood is more acid than that of. Introduction to buffer systems of blood: In this system, gaseous metabolic waste carbon dioxide reacts. Venous blood carries more co 2 than arterial blood. Identify the most powerful buffer system in the body. A buffer is a. Explain The Buffer System In The Blood.
From www.pinterest.ca
bicarbonate buffer system, example of multiple equilibria Teaching Explain The Buffer System In The Blood Introduction to buffer systems of blood: Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. Hence, the ph of venous blood is more acid than that of. Identify the most powerful buffer system in the body. A buffer is a solution that resists. Explain The Buffer System In The Blood.
From www.slideserve.com
PPT Blood Buffers PowerPoint Presentation, free download ID5687657 Explain The Buffer System In The Blood Hence, the ph of venous blood is more acid than that of. Identify the most powerful buffer system in the body. Explain the way in which the respiratory system affects blood ph. The ph of the blood is maintained between 7.35 and 7.45 by an important buffer system. A buffer is a solution that resists sudden changes in ph. The. Explain The Buffer System In The Blood.
From www.slideserve.com
PPT MLAB 2401 Clinical Chemistry Keri BrophyMartinez PowerPoint Explain The Buffer System In The Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Venous blood carries more co 2 than arterial blood. Identify the most powerful buffer system in the body. Hence, the ph of venous blood is more. Explain The Buffer System In The Blood.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 Explain The Buffer System In The Blood Introduction to buffer systems of blood: Hence, the ph of venous blood is more acid than that of. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Venous blood carries more co 2 than arterial blood. Explain the way in which the respiratory system affects blood ph. The buffer systems functioning in. Explain The Buffer System In The Blood.
From labpedia.net
Acidbase Balance Part 3 Respiratory Acidosis and Respiratory Explain The Buffer System In The Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Introduction to buffer systems of blood: Identify the most powerful buffer system in the body. A buffer is a solution that resists sudden changes in ph. Hence, the ph of venous blood is more acid than that of. The ph of the blood. Explain The Buffer System In The Blood.
From dxobuticl.blob.core.windows.net
Commonly Used Buffers In The Laboratory at Savannah Osgood blog Explain The Buffer System In The Blood Venous blood carries more co 2 than arterial blood. A buffer is a solution that resists sudden changes in ph. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. Identify the most powerful buffer system in the body. The ph of the blood. Explain The Buffer System In The Blood.
From www.slideserve.com
PPT Renal Physiology PowerPoint Presentation, free download ID5632772 Explain The Buffer System In The Blood In this system, gaseous metabolic waste carbon dioxide reacts. The ph of the blood is maintained between 7.35 and 7.45 by an important buffer system. Explain the way in which the respiratory system affects blood ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Identify the most powerful buffer system in. Explain The Buffer System In The Blood.
From www.slideserve.com
PPT MLAB 2401 Clinical Chemistry Keri BrophyMartinez PowerPoint Explain The Buffer System In The Blood Identify the most powerful buffer system in the body. The ph of the blood is maintained between 7.35 and 7.45 by an important buffer system. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. In this system, gaseous metabolic waste carbon dioxide reacts.. Explain The Buffer System In The Blood.
From facts.net
20 Fascinating Facts About Blood Buffer Explain The Buffer System In The Blood Venous blood carries more co 2 than arterial blood. Hence, the ph of venous blood is more acid than that of. The ph of the blood is maintained between 7.35 and 7.45 by an important buffer system. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Identify the most powerful buffer system. Explain The Buffer System In The Blood.
From ppt-online.org
Disorders of metabolism. (Subject 9) презентация онлайн Explain The Buffer System In The Blood Explain the way in which the respiratory system affects blood ph. Identify the most powerful buffer system in the body. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A buffer is a solution that resists sudden changes in ph. Hence, the ph of venous blood is more acid than that of.. Explain The Buffer System In The Blood.
From en.ppt-online.org
Blood biochemistry online presentation Explain The Buffer System In The Blood Venous blood carries more co 2 than arterial blood. In this system, gaseous metabolic waste carbon dioxide reacts. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Hence, the ph of venous blood is more acid than that of. The ph of the blood is maintained between 7.35 and 7.45 by an. Explain The Buffer System In The Blood.
From www.slideserve.com
PPT AcidBase Balance I. PowerPoint Presentation, free download ID Explain The Buffer System In The Blood In this system, gaseous metabolic waste carbon dioxide reacts. Introduction to buffer systems of blood: Identify the most powerful buffer system in the body. A buffer is a solution that resists sudden changes in ph. The ph of the blood is maintained between 7.35 and 7.45 by an important buffer system. The buffer systems functioning in blood plasma include plasma. Explain The Buffer System In The Blood.
From www.pinterest.com
Physiology Blood Buffer System Behrouz Human body facts Explain The Buffer System In The Blood Explain the way in which the respiratory system affects blood ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A buffer is a solution that resists sudden changes in ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Venous blood carries more. Explain The Buffer System In The Blood.